What Is the Correlation Between the pH and Absorption of Chemicals with Varing Concentration?
Info: 11025 words (44 pages) Dissertation
Published: 9th Dec 2019
Tagged: Chemistry
Literature review
What is the correlation between the pH and absorption of chemicals with varing concentration?
The rationale for chosing this topic is that it can be applied to everyday life, ie. Household items and food and the pH of them. For example, the ‘pH of tothpaste is approximately 9 and the pH of lemon juice is approximately 2.0’3. (ThoughtCo, 2018). The dangers of these household items can be quite severe used in large volumes, however when used in small volumes items such as lemon juice are not very harmful.
Acids
Acids are aqueous or gaseous substances that ‘Dissociate’ separate into simpler substances/atoms or molecules. An example of this is hydrogen chloride, which is a gas that has a ‘Covalent Structure’, this gas dissolves in water and forms hydrogen+ and chlorine– ions. The equation for this would be:
‘HCl(g) + H2O —- > H+(aq) + Cl–(aq)’
Hydrogen chloride gas + Water —- > Hydrogen+ ions + Chlorine– ions.
Hydrgoen was discovered to be an unstable element when it is alone and can simply be defined as a proton. In electrolysis reactions hydrogen ions combine with ‘Polar water molecules’ which in turn will then form stable ions called ‘oxonium ions’. An equation to show this is:
‘H+(aq) + H2O(l) —- > H3O+(aq)
Figure 1
Hydrogen + Water —- > Hydronium (RSC.org, 2018)
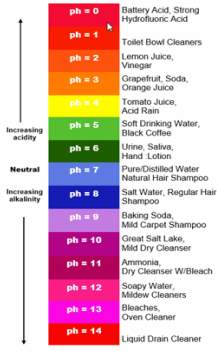
Figure 1 shows a diagram which highlights the pH scale and includes written examples of each pH.
(store,2018)
In addition, when hydrogen chloride as an aqueous solution is further dissolved in a volume of water, scientists have speculated that there is a chemical reaction taking place. The equation for the process is:
HCl(aq) + H2O(l) —- > H3O+(aq) + Cl–(aq)
Hydrogen chloride + Water —- > Hydronium + Chlorine (RSC.org, 2018)
The definition of an acid is: ‘A compound containing hydrogen that dissociates in water to form hydrogen ions’ (Annets et al., 2017).
Other common acids also react the same way, they dissociate and separate into simpler substances/ atoms or molcules, the amount they dissociate can vary from greater to lesser, but in water they all form oxonium ions. Therefore, this information explains why acids only portray their acidic properties when they have been diluted into a volume of water. (RSC.org, 2018).
Acids are substances that: form hydrogen gas when reacted with metals, neturalise bases and produce salt and water, and they release carbon dioxide if they are reacted with ‘Carbonates’. (RSC.org, 2018).
‘Acids provide H+ ions in a solution’. Acids can therefore be defined or described as ‘proton donors’. From this the assumption can be made that alkalis are ‘proton acceptors’ as they are chemical opposites to acids. (RSC.org, 2018).
Acid dissociation constant
The rate at which an acid dissociates varies with different acids. For example this could mean that more of the acidic molecules produce H+ ions and fhs ‘equilibrium position is further to the right’. An equation to show this for a general acid with the formula HA:
By following ‘Le Chatelier’s principle’, by adding more water to the acid , the ionisation of the acid will increase.
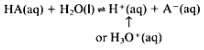
Therefore the ‘acid dissociation constant’ can be shown as this:
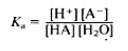
However, the concentration of the water being used is unlikely to vary very much as water is a neutral liquid, often around pH 7. Therefore, by taking this into consideration, the water constant is ‘incorperated’ into Kc, which can be shown in this equation:
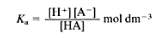
The values of
In this equation K can be defined as the ‘acid dissociation consatnt’
The ‘greater the degree of ionisation’ that is found, the stronger the acid will be. Therefore the value of K2 will increase.
When acids have the same concentration, the ‘greater the degree of ionisation’ of the acid being used, the more ions that are present in that acid. Therefore this will mean that the conductivity of the acid is higher. (RSC.org, 2018)
Some examples of acid dissociated constants are:
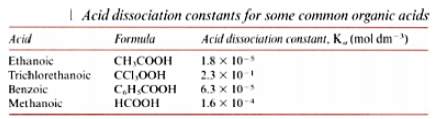
The reason that the value of Ka for trichloroethanoic acid is 10,000 times bigger than the value of Ka for ethanoic acid is because chlorine is an ‘electronegative atom’, this means that electrons are attracted to the chlorine nucleus. This weakens the O-H bond as the hydrogen ions are much easier to form. The more hydrogen ions found in a solution, the stronger the acidity of the solution.
Some examples of strong acids are sulfuric acid and nitric acids, these are common inorganic acids and they have high values of Ka as they acids are ‘fully ionised in a dilute solution’. (RSC.org, 2018)
pKa = -log Ka (RSC.org, 2018) OR pKa = -log Ka (Chemguide.co.uk, 2018)
The smaller the calculated or written value of pKa , the sronger the acidity of a solution.(RSC.org, 2018)
Ka has the units of: (mol dm-3) whereas, pKa does not have any units. (Chemguide.co.uk, 2018)
pKa is often used as it is easier to identify trends and patterns than using the Ka. (Chemguide.co.uk, 2018)
Examples of strong and weak acids:
Hydrochloric acid is a strong acid as in a dilute solution it is completely dissociated. An equation for this is:
HCl(aq) —- > H+(aq) + Cl–(aq)
Hydrochloric acid —- > Hydrogen + chlorine
However, in comparison both phenol and ethanoic acid can be described as weak acids as they never ‘completely ionised’ and therefore the dilution of the solution does not matter. Equations for both of these weak acids:
Phenol: C6H5H(aq) —- > C6H5O–(aq) + H+(aq)
Ethanoic acid: CH3COOH(aq) —- >CH3COO–(aq) + H+(aq)
There are many weak acids which are produced and occur naturally and therefore their origin is organic, such as:
- Methanoic acid- This is found in the string of an ant. This chemical was originally called formic acid.
- Ethanoic acid- This acid has a very strong smell. This chemical was originally called acetic acid.
- Butanoic acid- This acid off a smell of ‘rancid’ dairy products such as butter and cheese.
Strong acids are acids which are fully dissociated into simpler ions in a dilute solution.
Concentrated acids contain ‘serveral moles of substance’ for each dm3 of solution. For example, ‘ordinary lab’ hydrochloric acid has ‘approximately 10 mol dm-3 of HCl(aq). Another example is sulfuric acid which has approximately 18 mol dm-3 of aqueous H2SO4. (RSC.org, 2018)
Alkalis
Alkalis have a pH of 7-14, the higher the pH the stronger the alkalinity is of the solution. Weak alkalis, also referred to as soluble bases, such as ammonia have a pH of 10-11. However, strong alkalis, which are also referred to as soluble bases, such as sodium hydroxide have a pH of 13- 14. These pH levels show as blue/purple colours in solutions when using universal indicator. (Docbrown.info.,2018)
Alkalis form hydroxide ions (OH–(aq)) in water. Such as:
- Sodium hydroxide (NaOH) forms Na+(aq) and OH–(aq) ions.
- Calcium hydroxide Ca(OH)2 forms Ca2+(aq) and 2OH–(aq) ions.
‘An alkali is a base which is soluble in water’. (Docbrown.info.,2018)
Ionic theory of acids and alkalis
Liquid water consists of ‘covalent H2O moleucles’, it also contains small quantities of H+ and OH– ions from the ‘self-ionistion of water’. However, these ions are of equal concentration therefore, this makes the water neutral.
Acidic solutions contain more H+ ions than OH– ions. Alkalis are the opposite to acids, therefore alkali solutions contain more OH– than H+ ions. (Docbrown.info.,2018)
When acids and alkalis react the molecular formula equation is often for neutralisation:
Acid+ Alkali —- > Salt + Water Examples:
- HCl(aq) + NaOH(aq) —- > NaCl(aq) + H2O(l)
Hydrochloric acid + Sodium hydroxide —- > sodium chloride + water
However, the ionic equation for neutralisation reacts is:
Hydrogen ion + Hydroxide ion —- > water Which can be shown as:
H+(aq) + OH–(aq) —- > H2O(l)
This is because all when acids and water are reacted together it produces hydrogen ions. And when alkalis (soluble bases) and water are reacted together they produce hydroxide ions. Therefore, the remaining ions i.e. Na+(aq) and Cl–(aq) become salt crystals i.e. NaCl(s) when the salt ions evapourate from the water. (Docbrown.info.,2018)
‘Bases are substances which react and therefore neutralise acids to produce salts and water.’
Some bases are not soluble in water, examples of this include:
- CuO- Copper (II) Oxide
- MgO- Magnesium Oxide
After a neutralisation reaction, the ‘salt solutions consist of a combination of both positive and negative ions’. (Docbrown.info.,2018)
Brownsted-Lowry Theory
In this theory a ‘Brownstead-Lowry acid’ can be referred to as a ‘proton donor’. And as alkalis are the opposite, a ‘Brownstead-Lowry alkali’ can be referred to as a ‘proton acceptor’.
Water is a neutral oxifde as it has a pH of 7, however it is an ‘amphoteric oxide ’ as it accepts and donates protons.
If water acts like a base, a proton acceptor, ‘ reacted with a stronger acid such as the hydrogen chloride gas’, the equation for this is:
HCl(g) + H2O(l) —- > H3O+(aq) + Cl-(aq)
Hydrogen chloride gas + water —- > hydronium + chlorine
If water acts as an acid, a proton donator, reacted with a ‘weal but stronger base such as the alkaline gas ammonia’, the equation for this is:
 NH3(aq) + H2O(l) NH4+(aq) + OH-(aq) (Docbrown.info.,2018)
NH3(aq) + H2O(l) NH4+(aq) + OH-(aq) (Docbrown.info.,2018)
Indicators
‘Indicators are coloured dyestuffs’
There are many types of indicator such as: universal indicator, bromothylmol blue, methyl orange and phenolphthalein. (RSC.org, 2018)
In acids each indicator reacts differently:
- Universal indicator- red/pink
- Bromothylmol blue- yellow
- Methyl orange- red
- Phenolpthalein- colourless
In alaklis each indicator reacts differently once again, for example:
- Universal indicator- purple
- Bromothylmol blue- blue
- Methyl orange- yellow
- Phenolpthalein- pink
Universal indicator is also referred to as a ‘rainbow indicator’ or ‘mixed indicator’ as it as a combination of the single chemical indicators. Universal indicator is often used to find the pH of chemicals and a colour sheet or chart is used to match the colours. Some examples of different substances and the colour they are when universal indicator is added are: (RSC.org, 2018)
- Dilute hydrochloric acid- Red
- Boric acid solution- Yellow
- Tap/Drinking water- Green
- Sodium bicarbonate solution- Blue
- Sodium hydroxide solution- Purple
A comparison table to show the different indicators and the colour they change a chemical to, dependant on its pH.
(RSC.org, 2018)
| Bromothymol Blue | Methyl orange | Phenolphthalein | Universal indicator/ Mixed indicator | |
| Hydrochloric acid- HCL | Yellow | Red | Colourless | Red/Orange |
| Boric acid-
H3BO3 (Bibliography-7) |
Yellow | Yellow | Colourless | Yellow |
| Tap/ Drinking water- H2O | Green | Yellow | Colourless | Yellow/Green |
| Sodium bicarbonate-
NaHCO3 |
Blue | Yellow | Colourless | Blue/Green |
| Sodium hydroxide-
NaOH |
Blue | Yellow | Pink | Purple |
pH
pH ‘is a measure of the concentration of hydrogen ions in a solution’. The ‘higher the concentration of hydrogen ions in a solution, the lower the pH becomes.’ (Chemguide.co.uk, 2018).
It can be defined as:
pH = – log10 [H+]
The ‘log10’ should be read as ‘log to base 10’ (Chemguide.co.uk, 2018).
The square brackets for ‘[H+] mean the ‘the concentration in mol dm-3.
Methods of testing pH
The first researched method to test oxides is:
- 2cm3 of each oxide and water sample solution was placed into separate test tubes.
- 3 drops of universal indicator was added to each sample, the colour of each sample was then observed.
- The results were recorded in a table showing the name of the oxide the colour of the solution after the indicator was added, the pH, wether the oxide is acidic, alkaline or neutral in water.
(Rsc.org., 2018).
Another method is using a probe or meter to test the pH of a liquid:
- The probe was added to a buffer solution and the meter was adjusted accordingly.
- Rinse the pH probe with distilled water.
- The liquid is collected in a contained so the liquid level is deep enough to cover the tip of the probe.
- A thermometer was used to check the temperature of the sample, the meter (if it reads pH) was altered to match the temperature.
- The probe was placed into the sample and then after 30 seconds the measurement was taken. (Sciencing, 2018)
A second method for testing the pH of a liquid is:
- A sample of a liquid was collected in a clean container, making sure the sample is deep enough to cover the testing strip.
- The testing strip was dipped into the sample for a few seconds and then withdrawn to wait for the indicator bars to change colour.
- The end of the test strip is then compared with the colour chart in order to find the correct pH level of the liquid. (Sciencing, 2018)
A method for testing the pH of common household substances:
- A swab was used to collect saliva on the inside of a students mouth.
- The sample was then touched onto an unused pH testing strip.
Comparison of methods:
The first method I found (Rsc.org., 2018).highlighted a simple and accurate method of testing pH in oxides. It provides the reader with measurements of volumes, amounts of substance and clear instructions to follow.
The second method I found (Sciencing, 2018) provided a method to use a pH meter or probe to test the pH of a liquid. The method is simple and easy to follow, however it does not have specific volumes or measurements for the volume of liquid you need to use etc. However, it does provide you with a time frame you must wait to pass in order for the pH to reach its equilibrium and make it easier to read.
In addition, another method I found (Sciencing, 2018) had a simple and clear method to test the pH of a liquid using a pH testing strip. Alike the previous method, it did not have any specific measurements for the volume of liquid being used etc.
Another method I found (MnSTEP Activity Mini-collection., 2018) provided a very simplistic and basic experiment to test the pH of saliva. This method did not have any clear instructions, measurements or accurate descriptions you could follow.
The final method (Scientific research- open access, 2018) did not provide sufficient information on the topic, it just informed the reader of the buffer solutions which they made up and the calculated as well as measured pH of these substances. This reference did not actually provide a practical method.
Reactions of main group elements with water
Water or H2O consists of two hydrogen atoms and one water, as seen in the chemical formula. It ‘exhibits polarity’ and can be found in the liquid, solid and gaseous/vapour states naturally. Due to it being a polar substance it is therefore a ‘good solvent’, due to its universal use it is referred to as the ‘universal solvent’. Water is part of many chemical reactions and these reactions have trends which you can ‘catagorize using the periodic table’. (Chemistry LibreTexts., 2018)
Group 1: Alkali metals
Group one consists of alkali metals. The common characteritstic that the majority of most alkali metals have it ‘the ability to displace H2(g) from water/H2O’. This is shown by the alkali metals ‘large, negative electrode potenitals’. ‘In this event, the group 1 alkali metal is oxidised to its metal ion and water is reduced’, this produces hydrogen gas as well as hydroxide ions. An equation to show the general reaction of water/H2O(l) and an alkali metal(M),this equation is:
2M(s) + 2H2O(l) —- > 2M+(aq) + 2OH–(aq) + H2(g) (Chemistry LibreTexts., 2018)
As OH– is produced, it provides a ‘basic or alkaline environment’. The explanation for why group 1 elements are referred to as alkali metals is due to their ability to displace H2(g) from water and therefore produces a basic solution by doing this.
Alkali metals,’they react explosively as well as violently with water’, these are exthermic reactions as heat is released in order for the H2(g) to ignite in the water. Examples of alkali metals are Sodium (Na) and Lithium (Li). (Chemistry LibreTexts., 2018)
Alkali metal oxides and water:
When oxides of group 1 elements/ alkali metals react with water it produces a basic solution. When alkali metals react with oxygen they form different types of oxides which react with water in different ways, such as:
- Monoxides- M2O, these oxides produce ‘alkali metal hydroxides’, an equation to show this is:
M2O(s) + 2H2O(l) —- > 2M+(aq) + 2OH–(aq)
- Peroxides- M2O2, these oxides produce ‘metal hydroxides as well as hydrogen peroxide’, an equation to show this is:
M2O(s) + 2H2O(l) —- > 2M+(aq) + 2OH–(aq) + H2O2(aq)
- Superoxides- MO2, these oxides produce ‘metal hydroxides, hydrogen peroxide as well as oxygen gas’, an equation to show this is:
2MO2(s) + 2H2O(l) —- > 2M+(aq) + 2OH–(aq) + H2O2(aq) + O2(g)
Alkali metal hydrides and water
Alike group 1 oxides, group 1 hydrides react with water and produce a basic solution. However, ‘hydrogen gas is produced with the metal hydroxide’. An example of a general reaction for water and alkali metal hydrides is:
MH(s) + H2O(l) —- > M+(aq) + OH–(aq) + H2(g)
This reaction, shown above, ‘can be generalized to all of the alkali metal hydrides’.
Group 2: Alkaline Earth Metals
Most alkaline earth metals form hydroxides when they are reacted with water.The hydroxides of certain elements such as calcium, strontium and barium are not very soluble in water. Although, enough hydroxide ions are produced in the reaction to form a basic environment. An equation to represent the reaction of calcium, strontium and barium with water is: [M is strontium, calcium or barium in this case].
M(s) + 2H2O(l) —- > M(OH)2(aq) + H2(g)
An explanation for this equation is:
Magnesium reacts with water vapour in order to produce both magnesium hydroxide as well as hydrogen gas. Beryllium (Be) is the exception as it is the only alkaline ‘earth metal’ which does not react with water, this is because it is very small in size and yet its high ionisation energy in comparison to other elements in group 2.
Alkaline earth metal oxides and water
‘Alkaline earth metal monoxides, alike alkali metal oxides’ react with water in order to produce metal hydroxide salts. The general equation for this is: [The exception however is that beryllim, (the oxide is written as BeO), is not soluble and does not react with water.
MO(s) + H2O(l) —- > M(OH)2(s)
 CaO, also known as quicklime, is a very commonly known alkaline earth meetal oxide. It is as a way of treating water as well as ‘removing harmful SO2(g) from industrial smokestacks’.
CaO, also known as quicklime, is a very commonly known alkaline earth meetal oxide. It is as a way of treating water as well as ‘removing harmful SO2(g) from industrial smokestacks’.
Figure 2
(En.wikipedia.org, 2018)
Alkaline earth metal hydrides and water
Excluding beryllium, alkaline metal hydrides all react with water and form metal hydroxide as well as hydrogen gas. An equation to show the reaction of the metal hydrides can be shown as:
MH2(s) + 2H2O(l) —- > M(OH)2(aq) + 2H2(g) (Chemistry LibreTexts., 2018)
Group 13: Boron family
These elements are not very reactive in water, and boron itself does not react at all. However, when aluminium reacts with water there is no visible reaction as a thin layer of aluminium oxide forms around the metal in order to protect it, and therefore prevents the reaction. (Chemistry LibreTexts., 2018)
Group 14: Carbon family
Most of the group 14 elements do not react with water. Tin (Sn) is used as a protective layer over other metal cans ie iron in order to prevent corrosion. (Chemistry LibreTexts., 2018)
Group 15: Nitrogen Family
‘The pure elements in this group do not often react with water’. Compounds of the element nitrogen, i.e. nitrates and nitrites and nitrogen gas ‘dissolve in water but do not react’. (Chemistry LibreTexts., 2018)
Group 16: The oxygen family
‘Many of the group 1 and group 2 oxides react with water and produce metal hydroxides’. Nonmetal oxides react with water and produce something called an ‘oxoacid’. E.g. Phosphoric acid as well as the commonly used sulfuric acid. (Chemistry LibreTexts., 2018)
Group 17: Halogens
Usually when halogens react with water they produce halides and hypohalides. ‘Halogen gases vary in their reactions with water as they have different electronegatives’. I.e flurorine gas is so electronegative, it can ‘displace oxygen gas from the water’. The products of this reaction would then be oxygen gas as well as hydrogen fluoride. ‘The hydrogen halides reacts with the water to produce hydrohalic acids (HX).’ The exception of this would be HF, however the hydrohalic acids are still strong acids when added to water. An example of this using hydrochloric acid is:
Cl2(g) + 2H2O(l) —- > HCl(aq) + HOCl(aq)
‘Hypochlorus acid (HOCL) is a very strong bleaching agent and is not very stable when it is in a solution. Therefore, it decomposes, especially when in sunlight, yielding oxygen’.
2HClO —- > 2HCl + O2
(Chemistry LibreTexts., 2018)
When bromine liquid dissolves in water it turns it to a yellowish-brown solution.
Br2(g) + 2H2O(l) —- > HBr(aq) + HOBr(aq)
‘Hypobromous acid (HOBr) is a weak bleaching agent’.
Only a very small volume of iodine dissolves in water in order to produce a yellowish solution as well as hypoidous acid (HOI) which is a very weak bleaching agent due to its characteristics. (Chemistry LibreTexts., 2018)
Group 18: Noble gases
 Noble gases ‘do not react with water’. (Chemistry LibreTexts., 2018)
Noble gases ‘do not react with water’. (Chemistry LibreTexts., 2018)
Figure 3 (Chemistry LibreTexts., 2018)
Absoption spectra/ spectroscopy
 Spectroscopy is the ‘analysis of the interaction between matter and any portion of the electromagnetic spectrum’. It usually incolves the ‘visible spectrum of light’ however, ‘x-ray, gamma and UV spectroscopy are used as valuable analytical techniques’. (ThoughtCo., 2018)
Spectroscopy is the ‘analysis of the interaction between matter and any portion of the electromagnetic spectrum’. It usually incolves the ‘visible spectrum of light’ however, ‘x-ray, gamma and UV spectroscopy are used as valuable analytical techniques’. (ThoughtCo., 2018)
A beam of electromatic radiation is passed through a sample, the photons then interact with the sample and can do a number of things. Such as: be absorbed, reflected or refracted. The absorbed radiation ‘affects the electrons as well as the chemical bonds within the sample being tested’. (ThoughtCo., 2018)
The ‘absorbed radiation can lead to the emission of lower energy photons‘.(ThoughtCo., 2018)
Different types of spectra such as the emitted and absorbed spectra, can ‘be used to gain information about the material being tested’. (ThoughtCo., 2018)
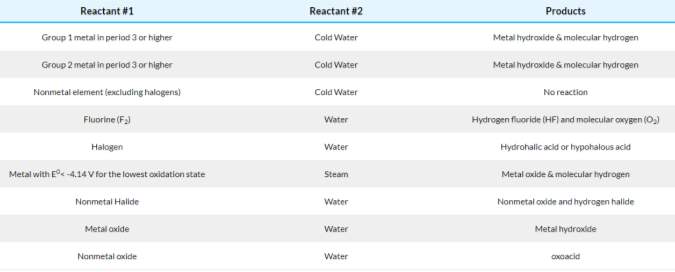
Figure 4
Figure 4 shows a ‘schematic diagram of a photoelectric colorimeter. The colour filter selected will only allow coloured light of that wavelength to pass through. I.e. a blue filter will allow a wavelength which is shown as the colour blue to pass through.
(Slideshare.net, 2018)
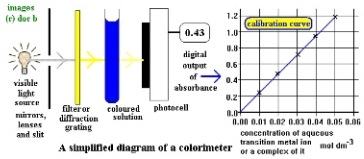
Figure 5
Figure 5 shows a ‘simplified diagram of a colorimeter’.
(Slideshare.net, 2018)
Method:
- The colorimeter was turned, on and left for 5 minutes to stabilise.
- The filter colour was selected, the filter chosen was and should be the opposite to the colour of the solution.
- A cuvette was filled with water, and the absorption was tested. This result is then referenced.
- The coloured solution was then added to a cuvette and placed into the colorimeter, the absorption is tested.
- Every so often, the water was re-tested to make sure the reference is correct.
(Techknow.org.uk, 2018)
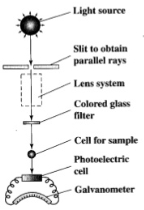
The filter you must choose should be the opposite colour to that of your sample, you can find this by comparing your sample to the colour wheel. The different filters prevent certain wavelengths of light from passing through.
Figure 6 shows the color wheel which is used to select a coloured filter when using a colorimeter. There are 2 types of filter and these are: gelatin filters as well as interference filters.
Gelatin filters ‘produce or transmit a wide band of radiation’, they are the more commonly used filter of the two.
Interference filters are used for accurate results as they have a ‘narrow bandpass, of approximately 10nm’. This type of filter is used to select certain wavelengths.
(Sherwood-scientific.com, 2018)
Initial researched references- summary
(Rsc.org.2018) is method for testing pH of oxides
(Sciencing.2018) is methods for testing pH of liquids
(ThoughtCo.2018) is pH of common chemicals
(MnSTEP Activity Mini-collection.2018) is method for testing pH of common household substances
(Rsc.org.2018) is teaching notes on acids and alkalis
(Oecd-ilibrary.org.2018) is a summary of a absorption spectra book
(Docbrown.info.2018) is more information on acids and alkalis
(Walker, D.,2007) is an online textbook on acids and alkalis
(Chemistry LibreTexts.2018) is reactions of main group elements with water.
(Scientific research- open access.2018) is an experiment testing pH and absorption using universal indicator.
Conclusion of literature review:
The literature review consisted of information on both pH as well as absorption spectroscopy, however I could not find research to link these topics. Therefore, I intend to use my practical experiment to identify any patterns or trends between the two sets of results, I will be able to see this by comparing two graphs.
However, thorough information was gained for pH as well as acids and alklais, there is not as much information on spectroscopy. To improve, there should be more detailed information in this topic to develop the readers knowledge of the topic.
Project proprosal
Introduction:
The practicle that will be carried out will show the relationship between pH and concentration as well and absorption and concentration. To conclude these two relationships will be compared to see if there are any changes, trends or patterns.
Coloured chemicals that will be used: [These coloured chemicals are to be used to test absorption in a colorimeter as well as pH by using a pH meter].
- Copper sulfate/ CuSO4(aq)
- Potassium manganate/ KMnO4(aq)
Colourless chemicals that will be used: [These chemicals are used to test pH using a universal indicator]
Non metals (Non-metal oxides dissolve in water to become acids):
Metals (Metal(basic) oxides dissolve in water to become alkalis):
- Sodium hydroxide/ NaOH(aq)
- Magnesium hydroxide/ MgOH(aq)
Hypothesis:
I think that the non-metals used will go down in pH as concentration goes up and that the metals will go up in pH as concentration goes up. In addition, I think that as concentration goes up the absorption will also go up, as the solution will become darker.
Equipment:
- Copper sulfate/ CuSO4(aq)– 0.5M, 1M, 1.5M, 2M
- Potassium manganite/ KMnO4(aq)– 0.5M, 1M, 1.5M, 2M
- Sulfuric acid/ H2SO4– 0.5M, 1M, 1.5M, 2M
- Hydrochloric acid/ HCl- 0.5M, 1M, 1.5M, 2M
- Sodium hydroxide/ NaOH(aq)– 0.5M, 1M, 1.5M, 2M
- Magnesium hydroxide/ MgOH(aq)– 0.5M, 1M, 1.5M, 2M
- Plastic pipettes
- Test tubes
- Colorimeter
- Univeral indicator
- pH meter
- Cuvette
- Distilled water
- Test tube rack
- Gloves
- Lab coat
- Goggles
- Light source (found in colorimeter)
- Electrisity source
- Beakers
Risk assessment:
| Hazard | Risk | Prevention |
| Trip hazard | Risk of falling over with or without chemicals or equipment and inuring either yourself or others. | Make sure all the stools are tucked away, as well as bags being pushed out of the way to prevent people from falling. |
| Slip hazard | Risk of people slipping on spilt liquid chemicals, that are clear or colourless. This could inure yourself or others, even if you were or weren’t carrying equipment. | Make sure to keep liquids away from the edge of the lab surface and prevent it from falling. If any chemical is spilt then make sure to clean it up instantly, and put up a wet floor sign if necessary. |
Using chemicals, i.e.
|
There are many risks of using chemicals such as them being:
Flammable Toxic Harmful An irritant Hazardous These chemicals can cause harm to the personal using them. |
In order to prevent harm from coming to yourself or others, make sure to be cautious and careful when transporting and using chemicals in the lab. Make sure you are wearing PPE, to decrease the risk of contact with the chemicals. |
Method:
pH 1: [I chose this method because it is simplistic and will allow me to develop my skills in using universal indicator. This method contains parts of previous methods I researched].
- A variety of chemicals were be selected, each chemical had a select few concentrations in order to create more reliable and detailed results.
- Approximately xcm3 of chemical A Concentration 1 was be added to a test tube using a pipette.
- The test tube was placed in the test tube rack.
- 2-4 drops of universal indicator was added to the solution.
- Using a pH level reference sheet, determine the approximate pH level of the chemical.
- This was then repeated for the rest of the concentrations.
- This process is then repeated for each chemical.
pH 2: [I chose this method because it is a simplistic way of using a pH meter and will allow me to develop the skills of using this piece of equipment. This method contains parts of previous methods I researched].
- The pH meter would be plugged in and the probe was left in its buffer solution.
- The probe was be removed from the buffer solution and washed briefly with distilled water.
- Approximately xcm3 of Chemical A Concentration 1 was be added to a beaker.
- The probe was be added to the beaker and left to rest for 30 seconds until the meter reaches equilibrium, the result was then be recorded.
- This was then be repeated for the rest of the concentrations.
- This entire process would then be repeated for each chemical.
Colourimetry/ spectroscopy method: [I chose this method as it is simplistic, it is also a combination of both my own practical knowledge as well as a researched method that I have used to construct this method].
- The colorimeter would be turned on.
- Using a pipette, a cuvette would be filled with approximately 5cm3 of water.
- A filter, which is the opposite colour of the chemical you wish to test, will be added to the colorimeter.
- The cuvette of water was placed into the colorimeter, and the absorption was tested. This number was then recorded/referenced.
- Another cuvette was filled with 5cm3 of Chemical A Concentration 1 and then was placed into the colorimeter, the absorption was tested and the result was then recorded.
- This was then repeated for each of the 4 concentrations.
- This process was then repeated for each chemical being tested.
Variables for pH and concentration:
Dependent variable- pH
Independent variable- concentration of the chemical
Controlled variables- Volume of chemical being used, amount of universal indicator being used, same equipment being used, the same universal indicator chart, amount of time you need to wait before using the pH meter, temperature.
Variables for absorbtion and concentration:
Dependent variable- absorbtion
Independent variable- concentration of the chemical
Controlled variables- Volume of chemical being used, same equipment being used, same reference solution being used, temperature.
Possible results:
Theory: metals produce alkalis and non-metals produce acids.
I predict that my results will prove this basic theory, and the stronger the concentration the stronger the acid or alkali will become.
Results:
Theoretically the results gained from both experiments will be plotted in tables, such as:
| Table to show the relatiobship between the concentration and pH of a variety of chemicals. | ||||||||||||
| Chemical | pH at concentration of 0.5M | pH at concentration of 1M | pH at concentration of 1.5M | pH at concentration of 2.5M | ||||||||
| 1 | ||||||||||||
| 2 | ||||||||||||
| 3 | ||||||||||||
| 4 | ||||||||||||
| Table to show the relatiobship between the concentration and absorption of a variety of chemicals. | ||||||||||||
| Chemical | Absorption (%Abs) at concentration of 0.5M | Absorption (%Abs) at concentration of 1M | Absorption (%Abs) at concentration of 1.5M | Absorption (%Abs) at concentration of 2.5M | ||||||||
| 1 | ||||||||||||
| 2 | ||||||||||||
| 3 | ||||||||||||
| 4 | ||||||||||||
Each section of results per concentration i.e. ‘pH at concentration of 0.5M’ or ‘Absoption (%Abs) at concentration of 0.5M’ are split into three as the experiment will have 3 trials in order to find an average as well as remove any anomalies.
Graphs:
The results for the experiment showing the relationship between the pH and concentration will be plotted in a graph, as shown below:
The results for the experiment showing the relationship between the absorption and concentration will be plotted in a graph, as shown below:
KEY: The units for the y-axis are %Absorbance.
Conclusion and evaluation:
Summary:
The methods which I formed for this plan were based on both my own knowledge as well as researched methods. I will be repeating each method 3 times in order to get an average result and therefore remove any anomalies as well as identify trends. Although, these trends will be easier to spot on a graph. I will be testing each method 3 times as it will allow my results to be more accurate and reliable, as well as repeatable for any other individual.
The tables I have produced allow my results to be recorded accurately and simply without causing confusion or over-complication of the results.
I will use the graphs formed by the results and compare them to one another to identify a common trend or pattern between them, which will then allow me to discuss the relationship between concentration & pH and concentration & absorption.
The risks I identified are common risks I have become aware of due to previous work in this scientific area, therefore my research allowed me to develop my knowledge rather than learning something new.
Potential limitations/sources of error:
- Systematic error- quantitive
Method:
If the volume of each chemical concentration isn’t kept the same then the results will be inconsistent and the trend/pattern will not be as clear and therefore the data will be effected.
If the amount of universal indicator isn’t kept the same then the strength of the colour will vary and this can lead to misinterpretation of a stronger or weaker pH than it is.
If the standard protocol for using each piece of equipment is not followed, this is general knowledge, then the personnel are at risk of both causing an accident as well as carrying out the experiment wrong.
Equipment:
pH meter– parallax error due to looking at the spidle at an angle and therefore misreading the result of the pH. To prevent this you could alternatively use a digital pH meter if they are available. If this piece of equipment is not calibrated properly it will not work, for example if the buffer solution the probe is kept in has been diluted, this would affect the pH levels you record. To prevent this make sure to test the buffer solution before hand or use a new solution.
Colorimeter– testing the transmittance instead of the absorption. Although this is a small error it could take away valuable time from the practical as well as creating anomalies and incorrect results.
Plastic pipette– if this piece of equipment is not sanitised and clean it could possibly cross contaminate the solution you pick it up with, this will therefore effect the numerical data i.e. concentration, pH and absorption. In addition, parallax could be an issue as the pipette may have internal stains preventing you from reading it correctly.
Test tubes– if the test tubes being used to hold a solution are unclean it will contaminate the solution within it and therefore the numerical data recorded will not be accurate, reliable or repeatable.
Universal indicator– if there is not a consistent amount of indicator added to each sample the results will be unreliable as some solutions will be much weaker in colour and therefore the pH will seem either less acidic or alkaline than it should.
Cuvette– if a cuvette being used is unclean not only will it contaminate the solution inside but it will also prevent light from passing through it, which is necessary. Which therefore, will cause all numerical data to be incorrect and unreliable.
Distilled water- if the distilled water is not checked for a pH, and has become contaminated it could also contaminate the equipment you clean with it. Therefore, this will effect the outcome of your experiment as the numerical results will not be correct.
Test tube rack-if the test tube rack is close to the edge of the lab surface there is a risk of it falling, also if the test tube rack is too big to hold the test tubes you risk causing an accident and if this occurs not only will someone be injured but your results will be effected.
Light source (in colorimeter)- if the bulb or cover over the light source is dirty or unclean it will prevent as much light from passing through and will therefore affect the numerical data, such as the absorption as the solution will not be able to absorb as much light as less light was emitted to begin with.
Electrisity source- if the electricity source ‘blows’ or stops working during the experiment, there is a risk of all the electrical dependent equipment needing to be recalibrated. Therefore, this will cost extra time which the personnel carrying out the experiment may not have. Also if the current from the electricity source is too high it may cause the electrical dependent equipment to break.
Beakers- if the beakers being used are unclean then there is a chance of misreading the result as well as contaminating the contents. Therefore the numerical data recorded will not be correct and the experiment will have to be repeated.
PPE (personal protective equipment)- if the personnel are not wearing PPE there is a risk of being harmed by the chemicals. Therefore due to this the personnel may misread a measurement due to unclean glasses etc.
- Random error
A random error in this experiment could be using varying amounts of indicator as that would cause the colour to be stronger in some and weaker than others which could cause misinterpretation as a weaker or stronger pH when that may not be the case.
Another example would be using the incorrect colour filter for the colorimeter, this would cause the colorimeter to be less effective and therefore the results will not be as valuable and may not show a clear trend or pattern which is comparable to that of the pH and concentration.
If you do not wait for the pH meter to settle when testing the pH of a solution, this takes approximately 30 seconds until it reaches its equilibrium, then the result may not be accurate as the needle on the pH meter will still be moving.
- Misreading of observations- qualitative
This type of error is a human error as it is the personnel reading the measurement, etc which is incorrect as no digital equipment is being used, excluding the colorimeter. Examples of this include:
pH meter– parallax due to looking at the arrow on the pH meter at the incorrect angle and therefore getting an incorrect and inaccurate result.
Pipettes- parallax due to the pipette being unclean or just a human error of reading the pipette from above rather than straight on.
beaker- there would be parallax due to human error of misreading it from the wrong angle, also if this piece of equipment is dirty it may be much harder to get an accurate measurement.
goggles- If the goggles being used are unclean it may prevent personnel from recording accurate and correct results, regardless if they look from head on or not. And as it would be too hazardous to take them off the only solution would be to use a clean, clear pair of goggles.
- Anomalous data
Causes:
If the personnel carrying out the experiment does not follow the method step by step, not only can this be hazardous but it can also cause the results to be incorrect as an important step could’ve been missed.
If parallax effects the results, or dirty equipment i.e. cuvettes causes numerical data to be inaccurate and therefore it may not be as easy to identify a trend.
Another cause is due to cross contamination of chemicals and the transfer of impurities, this can cause the results such as pH and absorption to be incorrect as their numerical value will not be the same with the added impurities.
Examples:
When you plot your results onto a graph it is much easier to spot anomalous data as it will not fit the pattern or trend that the other results follow. In addition, it will not follow the curve or line of best fit that the other results do.
To improve:
Equipment:
To improve I could use digital equipment which is not so reliant on calibration, and therefore I will only need to do this once and it will save time. This would also make my results more accurate and decrease the risk of parallax and therefore my results would be more reliable, accurate and repeatable.
In addition, I could make sure all my equipment is clean before using it, this will prevent cross contamination and reduce the risk of misreading a result due to unclean equipment.
Make sure all the equipment being used is well maintained and is in good working condition before using it.
Make sure both the buffer solution and distilled water are at the correct pH to prevent cross contaimation of pH levels.
Make sure there is a consistent amount of universal indicator being added to make it as much of a fair test as possible.
Before carrying out the experiment make sure all the googles, and other PPE are in good condition. The goggles should be clear and without markings across the eyepiece which could cause you to misread your results.
Method:
Make sure the method is very simplistic and clear with what to do, to reduce the risk of misunderstanding and measuring the wrong data etc.
Standards:
To make this experiment a ‘fair test’ the lab should be kept at room temperature, 21°C.
Strengths and weaknesses:
Accurary, reliability and precision:
Glossary:
| Term: | Definition |
| Aqeuous | ‘Like or containing water’
(Dictionary, 2018) |
| Polar | ‘A bond between two atoms where the electrons forming the bond are equally distributed’
(ThoughtCo, 2018) |
| Electronegative | ‘A measure of the tendency of an atom to attract a bonding pair of electrons’.
(Chemguide.co.uk, 2018) |
| Amphoteric | ‘Substance that can act as both an acid and a base’
(Annets et al., 2017) |
Results of literature search and review into the chosen area:
I have chosen to search and review into a chemistry topic.
The sources I have used provide sufficient information on topics such as:
- Testing the pH of oxides
- Methods for testing the pH of liquids
- pH of common chemicals
- pH of common household items
- Acids and alkalis
- Absorption spectra
- Acids, bases and salts
- Reactions of Main Group Elements with Water
- Absorbance vs. pH for a Universal pH Indicator
Reliability of the sources used:
Search engines:
- Google- Google is more of a generalised site which provides vast amounts of information ranging from videos to PDF publications, however the site often provides KS3 relevant resources unless specified otherwise which limits knowledge and details.
- GoogleScholar- This site is a educational site which specifically provides published documents relevant to the subject of study. However, it is slightly more difficult to use and does not always provide you with work which is relevant or useable.
Websites:
- RSC- Royal society of chemistry. This is a reliable source and will provide accurate and detailed information using scientific terminology. It may also reference other sources of information if you choose to look further into a subject.
- Sciencing- This site has references and detailed information which suggests it is an reliable source, it also has pictures as well as a video link which supports a variety of learning types.
- ThoughtCo- This site provides many standard pHs for household items and everyday objects which htherefore provideds insite to the dangers of pH in everyday lives. It seems like a reliable source, it is very simplistic however it does not provide references.
- Serc.carleton.edu- This is an educational site which his highlighted by the ‘.edu’ part of the link, this site is an academic source for teachers, therefore it is reliable.
- Oecd-ilibrary.org- A reliable source for information about the UV spectra for absorption.
- Docbrown.info- School source, this may not be as much of a reliable source. However, it does provide Alevel standard work on the topic of Acids, bases and salts.
- Walker, D. (2007). Acids and alkalis. London: Evans- This is a text book on acids and alkalis, this is a reliable source as it is an educational text book which has been published.
- Chemistry LibreTexts- Reliabile source which highlights the reactions of chemicals with water, I can use this as a way to select a variety of unreactive chemicals.
- Scientific research- open access- University level information, this is reliable and provides extensive results and research into the topic. This is the only relevant study I could find to the topic.
Bibliography: [Standard protocol- Harvard referencing]
| Title of proposed Research:
Testing the pH and absorption of different chemicals in the periodic table, varying in both concentration and group i.e. metals and non-metals. |
|||
| Word or search Term | Search Engine | Number of Hits | Most relevant references (full ref, Harvard style) |
| N/A | BTEC textbook | N/A |
|
| N/A | Google.co.uk | N/A |
|
| acid dissociation constant chemguide | Google.co.uk | Approximately
5,620 |
|
| Covalent structure definition | Google.co.uk | Approximately,
3,930,000 |
|
| Aqueous definition | Google.co.uk | Approximately,
38,900,000 |
|
| Testing acids and alkalis alevel | Google.co.uk | Approximately, 13,600,000 |
|
| Periodic table | Google.co.uk | Approximately 87,000,000 |
|
| Testing the pH of different chemicals | Google.co.uk | Approximately,
265,000,000 |
|
| Testing the absoption of different chemicals | Google.co.uk | Approximately, 54,900,000 |
https://www.oecd-ilibrary.org/environment/test-no-101-uv-vis-absorption-spectra_9789264069503-en [Accessed 17 Oct. 2018]. |
| Boric acid formula | Google.co.uk | Approximately ,
3,440,000 |
|
| Testing the pH of different chemicals | Google.co.uk | Approximately,
265,000,000 |
|
| Acids and alkalis | Google.co.uk | Approximately,
2,310,000 |
|
| Testing the pH of different chemicals | Google.co.uk | Approximately, 265,000,000 |
|
| N/A | Google.co.uk | N/A |
|
| Colorimetry colour wheel | Google.co.uk | Approximately,
59,100 |
|
| absorption spectroscopy colorimeter | Google.co.uk | Approximately 110,000 |
|
| pH scale | Google.co.uk | Approximately 181,000,000 |
|
| colorimeter method | Google.co.uk | Approximately,
496,000 |
|
| Covalent structure definition | Google.co.uk | Approximately,
3.930,000 |
|
| Testing the pH of different chemicals | Google.co.uk | Approximately, 265,000,000 |
|
| Spectroscopy | Google.co.uk | Approximately,
55,700,000 |
|
| Acids and alkalis | Scholar.google.co.uk | Approximately, 50,100 | BOOK:
Google Books. (2018). Acids and Alkalis. [online] Available at: https://books.google.co.uk/books? hl=en&lr=&id=nPnRO_mQymUC&oi=fnd&pg=PA4&dq=acids+and+alkalis&ots=w33OyZfmKT &sig=7y2v2qGO20and%20alkalis&f=false [Accessed 18 Oct. 2018] |
|
|
|||
|
|
|||
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Chemistry"
Chemistry is a science involving the study of the elements and matter at the atomic and molecular level including their composition, structure, properties, behaviour, and how they react or combine.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please: