Effect of Arsenic Poisoning on the Human Body
Info: 5877 words (24 pages) Dissertation
Published: 11th Dec 2019
Abstract
Throughout history, arsenic has been known as a poison that many people fear, however, humans are constantly exposed to this toxic element through air, water and soil. Most of this exposure does not pose health risks as there are mechanisms in place throughout the body to expel these small amounts of arsenic. In various places around the world, arsenic is more prevalent due to natural or human causes, which creates huge cause for concern as large amounts of arsenic can accumulate in the body leading to severe health problems. This review discusses the effect of arsenic poisoning on the human body and the mechanisms in place in the body to prevent and minimise these effects. The role of water pollution is also explored and the ways in which it can be reduced or rectified. The use of arsenic as an anticancer drug is also to discussed to establish whether arsenic can really be used effectively.
Table of Contents
3.2. Chronic arsenic poisoning
4.1. Sources of arsenic contamination
4.2. Prevention and clean-up of arsenic contamination
5.1. Historical therapeutic uses of arsenic
5.2. Current therapeutic uses for arsenic
1. Introduction
Arsenic is a toxic naturally occurring metalloid. Humans are constantly exposed to arsenic from natural sources. Both developed and underdeveloped countries around the world have arsenic present in their drinking water. In most cases, severe arsenic contamination is caused by arsenic-rich ores leaching arsenic into the groundwater. The problem is most severe in developing countries where chemical analyses of aquifers tend not be performed before wells are dug[1]. Figure 1 [2] shows arsenic contamination of ground water in various countries around the world. The areas the most affected areas are Bangladesh and West Bengal, India. Figure 1 estimates that estimates that approximately 30 million people in Bangladesh are exposed to water containing <1 to 2500
μ
g/L, which is up to 250 times more than acceptable levels of 10
μ
g/L set by the WHO[3]. This exposure to high levels of arsenic has been associated with a six-fold increase in still births in areas of Bangladesh and west Bengal where arsenic levels in water exceed 200
μ
g/L [4]. It has been linked to the increase of skin, lung and bladder cancer, all of which are commonly associated with arsenic toxicity[4].
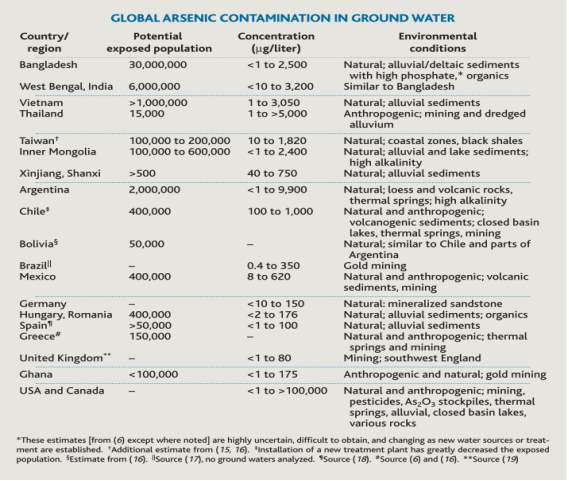
Figure 1. Table showing Global arsenic contamination in ground water[2]
Although arsenic is highly toxic it has been used for a variety of purposes throughout history. Arsenic was present in a pigment commonly used in the known 1800s as Paris green [5]. This pigment had been linked to many accidental arsenic poisonings as it was used in various products such as toys, candles and wallpaper before it was eventually phased out. An arsenic-containing drug developed by Thomas fowler was also the first chemotherapeutic agent used to treat leukaemia[6]. In the past, arsenic was often used for homicidal poisoning as it tasteless and odourless and therefore undetectable in food or drink[5]. Today arsenic is used in a variety of industries to make insecticides, fungicides, paints etc. The use of arsenic as a chemotherapeutic agent has also been explored further over the years to produce anticancer drugs used to treat cancer, specifically leukaemia[7].
2. Arsenic
2.1. Chemistry and Toxicity
Arsenic exists in both inorganic and organic forms. It is found primarily in rocks which when broken down release arsenic into air, soil and water[8]. It exists in three allotropic forms; yellow
(α)
, black
β
or grey
(γ)
form. Grey arsenic is the most common most stable form due to its double layer structure and is a brittle semi-metal. Arsenic has various oxidation states, the most common oxidation states being -3, +3 and +5 [9]. The toxicity of arsenic varies with oxidation state, whether arsenic is the inorganic or organic form, its solubility etc. Inorganic forms of arsenic are more toxic than organic forms of arsenic as they are more rapidly absorbed. The most toxic forms of inorganic arsenic are the trivalent (As III) and pentavalent (As V) with trivalent inorganic arsenic being 60 times more toxic than pentavalent arsenic [10].
Pentavalent arsenic (arsenate, AsO43-) can replace phosphate ( PO4) due to its similar structure and properties, which in turn affects a number of biochemical reaction [7]. Arsenate is metabolised in the mitochondria and can affect oxidative phosphorylation by binding to the Fo/F1 ATP synthase, an enzyme that creates ATP[7]. The arsenate is used more efficiently by ATP synthase and produces ADP-arsenate which hydrolyses much quicker than ADP-Phosphate (ATP)[11]. This reduces the formation of ATP by replacing phosphate with arsenate in enzymatic reactions. Trivalent arsenic (arsenite, AsO33- ) is much chemically reactive that arsenate which is why it is more toxic. Mechanisms transport the arsenite into the cell where it can be oxidised to arsenate to reduce toxicity [12]. Arsenic also binds to thiols and proteins that contain thiol groups. Arsenic toxicity needs to be investigated in greater depth as the mechanisms are not fully known. Many studies test arsenic toxicity by studying the effects on animals. However, the same results are often not seen in humans. By better understanding mechanisms of arsenic toxicity, both chronic and acute arsenic poisoning can be better treated and prevented.
2.2. Metabolism
The main method of metabolism of arsenic is biomethylation. This converts inorganic arsenic into non-toxic organic arsenic as organoarsenic compounds are unlikely to pose a hazard. In mammals, inorganic arsenic in methylated to monomethylarsonic acid (MMA) and dimethylarsinic acid (DMA)[8], [13]. The methyl done is S-adenosyl-methionine (SAM) and the reaction is catalysed by methyltransferase[8], [14].
Metabolism by methylation can be affected by various factors such as diet and organ function. Diets containing low levels of methyl groups have been found to be linked to lower arsenic methylation[13]. Fat is also thought to affect the methylation process as it can limit the amount of arsenic that is absorbed and metabolised[15]. High-fat diets have been linked to liver damage and could cause hepatic oxidative stress and liver fibrogenesis[15].Liver function is also important in arsenic methylation as it is thought to be the main site of arsenic methylation[16].Studies have found that arsenic methylation improves when end-stage liver disease patients receive liver transplants[13]. Metabolism can also vary with age, one study found that children in rural Bangladesh whose mothers had been exposed to arsenic during pregnancy had a higher percentage of DMA and a lower percentage of inorganic in their urine compared to their mothers[17]. Selenium and folate are strongly related to methylation in the children unlike in their mother.
Methylation occurs when arsenic is in its trivalent form, so the reduction of pentavalent arsenic to trivalent arsenic is a key step in the methylation process and is thought to occur largely in the blood. Trivalent arsenic is more toxic than pentavalent arsenic so it could be assumed that the reduction of As(V) to As(III) increases arsenic toxicity, however, the majority of As(III) is absorbed by bodily tissues and undergoes methylation. Figure 2 [12] shows how inorganic arsenic is metabolised and transformed in mammals.
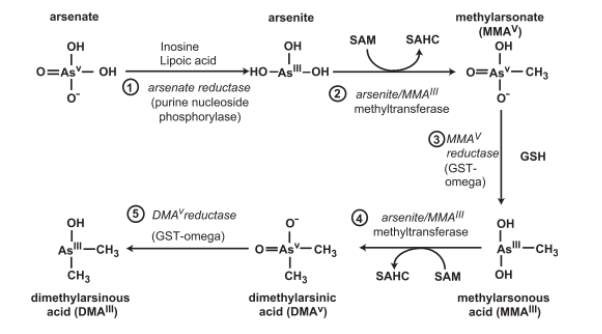
Figure 2. Biotransformation of inorganic arsenic in mammalian systems. SAHC, S-adenosylhomocysteine [12]
The levels of MMA and DMA found in urine are used to determine the efficiency of methylation[18]. Secondary methylation is very important as higher levels of MMA in urine has been linked to high incidence of arsenic-related health problems. One study found that in the Pabna district of Bangladesh, people with skin lesions, a symptom of arsenic poisoning, were more likely to have a higher proportion of MMA and a lower proportion of DMA in their urine [19].This shows that the incidence of arsenic-related diseases is not only affected by the exposure of individuals to arsenic but is also affected by the efficiency of arsenic metabolism.
3. Health effects
3.1. Acute arsenic poisoning
Acute arsenic poisoning is when a large single dose of arsenic leads to severe symptoms. It is usually linked with accidental, homicidal or suicidal poisoning. Symptoms can occur as quickly as 30 minutes of ingestion[20]. The affected individual may experience a metallic taste or a slight garlicky odour and difficulty in swallowing. Small amounts, less than 5mg, results in vomiting and diarrhoea but will subside in 12 hours[10]. Recent non-lethal doses of arsenic have also been linked to a small decrease in intellectual testing[21]. Lethal doses, depending on the exact amount of arsenic consumed, can cause death within 24hours to 4 days[10].
Muscular pain and weakness are some of the first symptoms to be observed shortly followed by severe nausea, vomiting, dehydration and diarrhoea[22]. The haematological system is damaged leading to common effects such as Anaemia, leukopenia and bone marrow depression[10], [20]. The collapse of the circulatory system can lead to cold clammy skin. Renal failure has also been a reported symptom. The neurological system is also affected and drowsiness, confusion and psychosis are often seen[20]. The most common neurological system is peripheral neuropathy[10]. Peripheral neuropathy is damage to nerves causing impaired sensation, movement gland or organ function, it can often be a delayed symptom[22] and can last up to two years or more. In the final stages, seizures can occur as well as coma’s and then finally death[20].
3.2. Chronic arsenic poisoning
Chronic arsenic poisoning occurs when repeated doses of arsenic compounds over a prolonged period leading to an accumulation of arsenic in the body. Most people are chronically exposed to arsenic through drinking water. In Bangladesh, more than 30 million people are exposed to arsenic-contaminated drinking water. One study conducted in Araihazar, Bangladesh estimated 21.4% of all deaths and 23.5% of deaths associated with chronic population could be accredited to exposure to high levels of arsenic through drinking water[23]. The same conclusions have been drawn in various regions with severe levels of arsenic contamination in water such as West Bengal, Vietnam and Mongolia.
The early symptoms usually affect the skin making it hard to diagnose as there are many causes of skin irritation. The skin firstly appears to have some redness which eventually progresses into a variety of skin problems such as; melanosis, hyperkeratosis and desquamation[20].Hyperpigmentation can also occur giving a raindrop appearance due to the numerous rounded hyperpigmented macules around the body[24]. These skin symptoms progress over time before more serious internal symptoms develop. Common symptoms at this stage affect the lungs and can result in problems such as asthmatic bronchitis. Other organs such as the liver, muscles, eyes and vessels are also effected[20]. If the affected individual survives these various complications cancer tends to develop. Cancer usually does not develop until years of arsenic exposure with the most common forms associated with arsenicosis being skin, lung and bladder cancer[25].
Arsenic passes through the human placenta so prolonged expose to arsenic can result in complications throughout pregnancy. Chronic arsenic poisoning has also been linked with fetal loss, premature pregnancy and spontaneous abortion. One of the largest studies conducted on fetal loss in Matlab, Bangladesh found drinking water with more than 50
μ
g of arsenic per litre for the duration of pregnancy increased risks of fetal loss[26]. Reductions in birth weight have also been associated with chronic arsenic poisoning in Taiwan and Bangladesh[27]. The effects of arsenic exposure during pregnancy have also been linked health problems in childhood. One study found a 69% increased risk of Lower respiratory tract infection and a 20% increased risk of diarrhoea during childhood[28].
4. Arsenic Contamination
4.1. Sources of arsenic contamination
4.1.1. Natural sources
In general, most cases of chronic acute poisoning are caused by drinking arsenic-contaminated water. Many cases of arsenic contamination come from natural causes. Arsenic is found in more than 200 minerals such as elemental As, arsenide’s, sulphides, oxides, arsenates and arsenites[29]. Most are Ore minerals with the most abundant ore mineral being arsenopyrite, FeAsS[30], it is formed under high-temperature conditions in the earth crust. As these minerals and ores are broken down arsenic seeps into the aquifer and hence the groundwater. Arsenic also enters the atmosphere by wind erosion, volcanic emissions, low-temperature volatilization from soils, marine aerosols and pollution[30] and is then returned to the earth’s surface.
Examples of these natural occurrences of arsenic contamination are in Inner Mongolia, Vietnam and West Bengal. In Inner Mongolia, arsenic exposure occurs from natural origins. There is high arsenic content in the ores found in the Yinshan mountains[31] and the rain precipitates arsenic downwind into the groundwater. In Vietnam, the Red River that served the capital of Hanoi and the rural areas surrounding it. In the rural area, the population has been growing rapidly with the population largely moving away from the use of surface water in favour of groundwater. The Red river contains large quantities of silt, rich in iron oxide and is anoxic leading to high levels of arsenic, with the average being 159
μ
g/L[32].
4.1.2. Anthropogenic Sources
Two-thirds of the As emissions to the atmosphere are due to anthropogenic sources, particularly from industrial sources. Figure 3 shows the cumulative production of As from mining, coal and petroleum[33]. It shows a dramatic increase at the beginning of the 20th century.
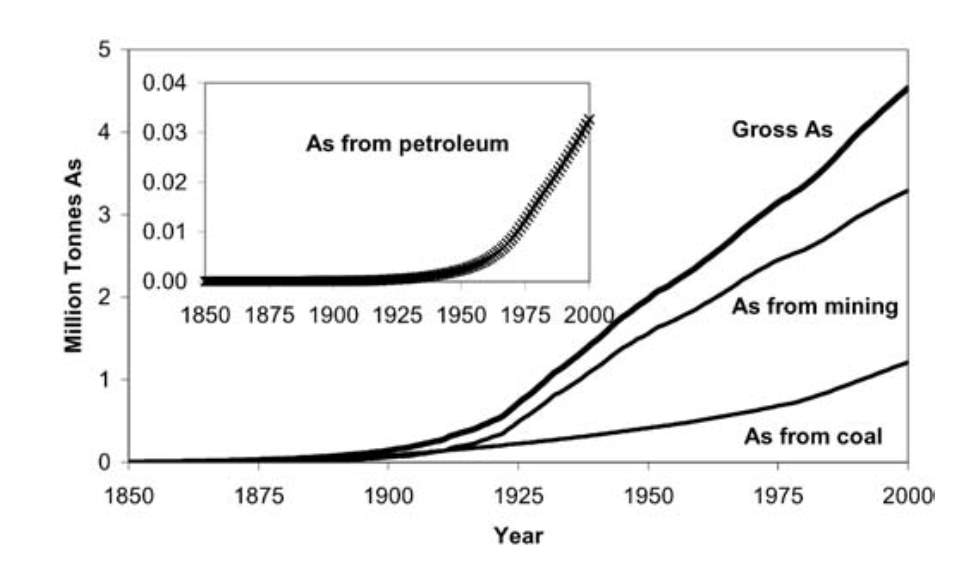
Figure 3. Graph showing the cumulative production of Arsenic from Coal, Petroleum and Mining[33]
The main sources of Arsenic emissions are metal production and the burning of fossil fuels such as coal[34]. Although arsenic exists in over 200 minerals only a few minerals contain substantial amount of arsenic. Copper production is the main source of anthropogenic emission of As, due to mining and formation of metallic sulphides by Cu smelting and refining[34]. Pyrites such as arsenopyrite can contain up to 10% in weight of arsenic that can be released with burning the pyrites.
The burning of Coal is the second main source of Arsenic emission. Most coal contains low concentrations of arsenic so poses no risk to humans (less than 5mg kg-1)[35]. But some coals contain large amounts of arsenic and may contain up to 35000 mg kg-1[35]. The arsenic is volatilized from the burned coal and hence released into the atmosphere.
Many power plants in countries such as India[35], Slovakia[36] and China[33] may be a major source of pollution in the environment. Many of these plants produce waste water that is highly contaminated with arsenic that is not properly disposed of[35]. This contributes to the prevalence of chronic arsenic poisoning by the inhalation of arsenic in the air and consumption of contaminated food and water.
Other anthropogenic sources of Arsenic come from products containing Arsenic such as herbicides, pesticides, wood preservatives, semiconductors and metal alloys and glass manufacturing[37]. Agricultural uses of arsenic accounted for a large amount of arsenic use in the 1940s[38]. However, it was phased out later in the 20th century due to discoveries pesticide run off was contaminating rivers and streams, as well as posing a serious health risk to farm workers. Arsenic was still used in wood preservatives[37], [38] but efforts are being made to reduce the use arsenic-containing products in manufacturing. Some developing countries continue to use these arsenic-containing products.
4.2. Prevention and clean-up of arsenic contamination
Arsenic contamination is becoming an increasing problem all over the world, leading to severe health and environmental problems. To prevent the effects of arsenic contamination
5. Arsenic Based drugs
5.1. Historical therapeutic uses of arsenic
Although arsenic has been infamous throughout history due to its poisonous nature, it has been used in medicine for over 2000 years. Traditional Chinese medicine(TCM) has a common concept of fighting poison with poison and the use of arsenic in TCM can be dated as far back as 200 BC during the Han Dynasty[39]. Two forms of arsenic were mainly used, arsenic sulphide, referred to as realgar and arsenic trioxide referred to as pishuang. Realgar was used to treat chills, jaundice and ulcers as well as various other ailments[39]. Arsenic was also used in Indian Ayurvedic herbal medicines along with lead and mercury, which are other known poisonous metals[40]. Ayurvedic herbal medicines were thought to be made from “essence of five planets” and people believed they could give everlasting life.
In the west, Hippocrates used arsenic sulphides as well as realgar and orpiment[6], [40] to treat ulcers and abscesses in the same way it was being used in the east. In the 16th Paracelsus, an Italian physician and professor of medicine, was the first to document the preparation of arsenic as a therapeutic drug and created a balsam that was used to treat wounds and ulcers[40]. The following century Thomas Fowler developed a solution containing 1% potassium arsenite to treat fevers and headaches[41], [42]. The solution became known as Fowler’s solution and was used to treat a variety of ailments in the 19th century such as malaria, asthma and hypertension. In the late 19th century it was discovered that Fowler’s solution could reduce the white blood cell count in leukaemia[40]. It then became the primary treatment for leukaemia until the development of radiation and chemotherapy as the side effects of fowlers solution were far greater.
In the early 20th century Paul Ehrlich began experimenting with arsenic compounds for treating trypanosomiasis (African sleeping sickness) and syphilis. In 1909, he successfully formulated dioxy-diamino-arsenobenzol-dihydrochloride referred to as drug”606”[43]. This later led to the development of arsphenamine which was the first effective treatment for syphilis and was used to treat it until the development of penicillin in the 1940’s. Arsphenamine is regarded as the first modern chemotherapeutic drug and Ehrlich went on to win the Nobel prize for this discovery.
5.2. Current therapeutic uses for arsenic
Arsenic was used to treat leukaemia after the discovery that it reduced white blood cell count[40], however, treating leukaemia this way was phased out with the development of chemotherapy and radiation treatments[44]. Interest in the development and use of arsenic and arsenicals was almost non-existent for a large part of the 20th century. But in the early 1970’s, Chinese research scientists identified a solution of As2O3 [44], [45]with trace amount of mercury based on TCM as a treatment for acute promyelocytic leukaemia (APL).
APL is a subtype of Acute myeloid leukaemia (AML) that accounts for approximately 10-15%[46]of AML cases. APL is characterised by an abnormal accumulation of promyelocytes caused by the chromosomal translocation between chromosomes 15 and 17[47]. This results in the fusing of retinoic acid receptor alpha (RAR
α
) and the promyelocytic leukaemia gene to give a fusion protein PML-RAR
αADDIN CSL_CITATION { “citationItems” : [ ], “properties” : { “noteIndex” : 0 }, “schema” : “https://github.com/citation-style-language/schema/raw/master/csl-citation.json” }
[48]. In the past, APL was treated with standard AML treatments with approximately70-80%[48] of patients going into complete remission (CR). However, over half of those in remission eventually relapsed. Later, All-trans-retinoic acid (ATRA) was introduced to treat APL. ATRA targets the retinoic acid receptors and induces differentiation of APL blasts[48] (See Figure 4) and improved CR in over 90% of case after approximately 4 to 5 weeks[47].
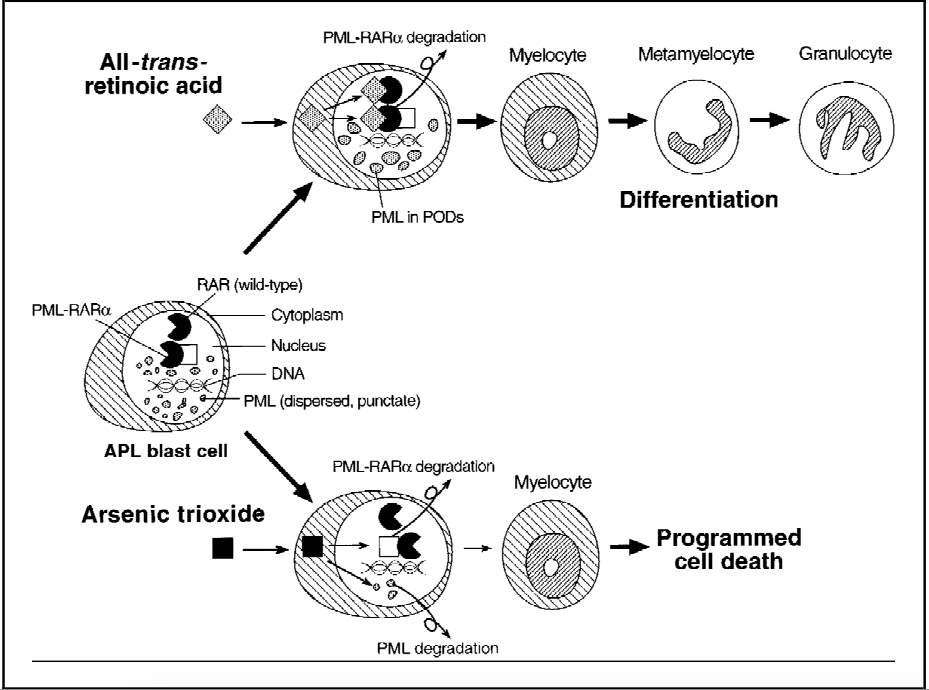
Figure 4. Figure showing the effects of ATRA and As2O3 on PML-RAR
α
and other nuclear proteins in the blast cells of APL[49]
As2O3 has been shown to be active against APL that has been resistant against ATRA[46]. As2O3 has been shown to be a gene-targeted treatment as it treats APL by degrading the fusion protein, PML-RAR
α
(See Figure 4). After being treated with As2O3, those who had been previously resistant to ATRA have been seen to have a remission rate of 83-95%[50]. One study found that of 15 people treated with As2O3, 14 achieved CR[51]. However, the same study found that the use of As2O3 resulted in side effects. Common side effects included loss of appetite, dermatologic symptoms and gastrointestinal symptoms as well as ECG changes. Although both ATRA and As2O3 have high remission rates when used alone, it has been found that when they used in conjunction with one another they work more effectively. A clinical trial based in china tested the use of ATRA, As2O3 and ATRN/ As2O3[52], found that the combined used of ATRA and As2O3 had better results resulting in fewer relapses and faster CR.
References
[1] T. Gebel, “Confounding variables in the environmental toxicology of arsenic,” Toxicology, vol. 144, no. 1–3, pp. 155–162, 2000.
[2] D. K. Nordstrom, “Worldwide Occurrences of Arsenic in Ground Water,” vol. 296, no. June, pp. 64–65, 2002.
[3] WHO, “Exposure to Arsenic: A Major Public Health Concern,” Agriculture, p. 5, 2010.
[4] D. Guha Mazumder and U. B. Dasgupta, “Chronic arsenic toxicity: Studies in West Bengal, India,” Kaohsiung J. Med. Sci., vol. 27, no. 9, pp. 360–370, 2011.
[5] M. F. Hughes, B. D. Beck, Y. Chen, A. S. Lewis, and D. J. Thomas, “Arsenic exposure and toxicology: A historical perspective,” Toxicol. Sci., vol. 123, no. 2, pp. 305–332, 2011.
[6] D. M. Jolliffe, “A history of the use of arsenicals in man.,” J. R. Soc. Med., vol. 86, no. 5, pp. 287–289, 1993.
[7] S. J. Ralph, “Arsenic-based antineoplastic drugs and their mechanisms of action,” Met. Based. Drugs, vol. 2008, 2008.
[8] P. Roy and A. Saha, “Metabolism and toxicity of arsenic: A human carcinogen,” Curr. Sci., vol. 82, no. 1, pp. 38–45, 2002.
[9] K. Jomova et al., “Arsenic: Toxicity, oxidative stress and human disease,” J. Appl. Toxicol., vol. 31, no. 2, pp. 95–107, 2011.
[10] R. N. Ratnaike, “Acute and chronic arsenic toxicity,” pp. 391–397, 2003.
[11] M. F. Hughes, “Arsenic toxicity and potential mechanisms of action,” Toxicol. Lett., vol. 133, no. 1, pp. 1–16, 2002.
[12] H. Vasken Aposhian, R. A. Zakharyan, M. D. Avram, A. Sampayo-Reyes, and M. L. Wollenberg, “A review of the enzymology of arsenic metabolism and a new potential role of hydrogen peroxide in the detoxication of the trivalent arsenic species,” Toxicol. Appl. Pharmacol., vol. 198, no. 3, pp. 327–335, 2004.
[13] M. Vahter, “Mechanisms of arsenic biotransformation,” Toxicology, vol. 181–182, pp. 211–217, 2002.
[14] B. L. Pierce et al., “Arsenic metabolism efficiency has a causal role in arsenic toxicity: Mendelian randomization and gene-environment interaction,” Int. J. Epidemiol., vol. 42, no. 6, pp. 1862–1872, 2013.
[15] H. Yu, S. Liu, M. Li, and B. Wu, “Influence of diet, vitamin, tea, trace elements and exogenous antioxidants on arsenic metabolism and toxicity,” Environ. Geochem. Health, vol. 38, no. 2, pp. 339–351, 2016.
[16] K. Rehman and H. Naranmandura, “Arsenic metabolism and thioarsenicals,” Metallomics, vol. 4, no. 9, p. 881, 2012.
[17] H. S. L??veborn et al., “Arsenic metabolism in children differs from that in adults,” Toxicol. Sci., vol. 152, no. 1, pp. 29–39, 2016.
[18] C. Tseng, “Arsenic Methylation, Urinary Arsenic Metabolites and Human Diseases: Current Perspective,” Perspective, vol. 501, no. July 2011, pp. 37–41, 2007.
[19] M. L. Kile et al., “A pathway-based analysis of urinary arsenic metabolites and skin lesions,” Am. J. Epidemiol., vol. 173, no. 7, pp. 778–786, 2011.
[20] J. C. Saha, a K. Dikshit, M. Bandyopadhyay, and K. C. Saha, “A review of arsenic poisoning and its effects on human health,” Crit Rev Env. Sci Technol, vol. 29, no. July 1999, pp. 281–313, 1999.
[21] O. S. von Ehrenstein et al., “Children’s intellectual function in relation to arsenic exposure,” Epidemiology, vol. 18, no. 1, pp. 44–51, 2007.
[22] A. Vahidnia, G. B. van der Voet, and F. A. de Wolff, “Arsenic neurotoxicity : A review,” Hum. Exp. Toxicol., vol. 26, no. 10, pp. 823–832, 2007.
[23] M. Argos et al., “Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): A prospective cohort study,” Lancet, vol. 376, no. 9737, pp. 252–258, 2010.
[24] G. M. D.N., “Chronic arsenic toxicity & human health,” Indian J. Med. Res., vol. 128, no. 4, pp. 436–447, 2008.
[25] S. Kapaj, H. Peterson, K. Liber, and P. Bhattacharya, “Human Health Effects from Chronic Arsenic Poisoning – A Review,” Jounral Environ. Sci. Heal. Part A, vol. 41, no. April, pp. 2399–2428, 2006.
[26] A. Rahman et al., “Association of arsenic exposure during pregnancy with fetal loss and infant death: a cohort study in Bangladesh.,” Am. J. Epidemiol., vol. 165, no. 12, pp. 1389–96, 2007.
[27] A. H. Smith and C. M. Steinmaus, “Health Effects of Arsenic and Chromium in Drinking Water: Recent Human Findings,” pp. 107–122, 2010.
[28] A. Rahman, M. Vahter, E.-C. Ekström, and L.-Å. Persson, “Arsenic exposure in pregnancy increases the risk of lower respiratory tract infection and diarrhea during infancy in Bangladesh.,” Environ. Health Perspect., vol. 119, no. 5, pp. 719–24, 2011.
[29] W. Q. Chen, Y. L. Shi, S. L. Wu, and Y. G. Zhu, “Anthropogenic arsenic cycles: A research framework and features,” J. Clean. Prod., vol. 139, pp. 328–336, 2016.
[30] P. L. Smedley and D. G. Kinniburgh, “A review of the source, behaviour and distribution of arsenic in natural waters,” Appl. Geochemistry, vol. 17, no. 5, pp. 517–568, 2002.
[31] J. X. Guo, L. Hu, P. Z. Yand, K. Tanabe, M. Miyatalre, and Y. Chen, “Chronic arsenic poisoning in drinking water in Inner Mongolia and its associated health effects.,” J. Environ. Sci. Health. A. Tox. Hazard. Subst. Environ. Eng., vol. 42, no. 12, pp. 1853–1858, 2007.
[32] M. Berg, H. C. Tran, T. C. Nguyen, H. V. Pham, R. Schertenleib, and W. Giger, “Arsenic Contamination of Groundwater and Drinking Water in Vietnam: A Human Health Threat,” Environ. Sci. Technol., vol. 35, no. 13, pp. 2621–2626, 2001.
[33] F. X. Han, Y. Su, D. L. Monts, M. J. Plodinec, A. Banin, and G. E. Triplett, “Assessment of global industrial-age anthropogenic arsenic contamination,” Naturwissenschaften, vol. 90, no. 9, pp. 395–401, 2003.
[34] D. Sánchez-Rodas, A. M. S. de la Campa, and L. Alsioufi, “Analytical approaches for arsenic determination in air: A critical review,” Anal. Chim. Acta, vol. 898, pp. 1–18, 2015.
[35] J. C. Ng, J. Wang, and A. Shraim, “A global health problem caused by arsenic from natural sources,” Chemosphere, vol. 52, no. 9, pp. 1353–1359, 2003.
[36] V. Bencko, “Health aspects of burning coal with a high arsenic content: The central Slovakia experience.,” Abernathy, C.O., Calderson, R.L., Chappell, W.R., eds., Arsen. Expo. Heal. Eff. London, Chapman Hall, vol. 395, pp. 84–92, 1997.
[37] R. J. Bowell, C. N. Alpers, H. E. Jamieson, D. K. Nordstrom, and J. Majzlan, “The Environmental Geochemistry of Arsenic — An Overview –,” Rev. Mineral. Geochemistry, vol. 79, no. 1, pp. 1–16, 2014.
[38] S. Wang and C. N. Mulligan, “Effect of natural organic matter on arsenic release from soils and sediments into groundwater,” Environ. Geochem. Health, vol. 28, no. 3, pp. 197–214, 2006.
[39] W. Y. Au, “A biography of arsenic and medicine in Hong Kong and China,” Hong Kong Med. J., vol. 17, no. 6, pp. 507–513, 2011.
[40] J. Frith, “Arsenic – the ‘ Poison of Kings ’ and the ‘ Saviour of Syphilis ,’” J. Mil. Veteran’s Heal., vol. 21, no. 4, pp. 11–18, 2013.
[41] K. H. Antman, “Introduction: The History of Arsenic Trioxide in Cancer Therapy,” Columbia Univ. Coll. Physicians Surg., vol. 6, no. suppl 2, pp. 1–2, 2001.
[42] Cullen WR, “Medicinal Arsenic : Toxic Arsenic,” Is Arsen. an Aphrodisiac? Sociochemistry an Elem., pp. 1–55, 2008.
[43] J. Frith, “Syphilis – Its early history and treatment until penicillin, and the debate on its origins,” J. Mil. Veterans. Health, vol. 20, no. 4, pp. 49–58, 2012.
[44] P. J. Dilda and P. J. Hogg, “Arsenical-based cancer drugs,” Cancer Treat. Rev., vol. 33, no. 6, pp. 542–564, 2007.
[45] A. M. Pizarro et al., “Medicinal Organometallic Chemistry,” Top Organomet Chem, vol. 32, no. April 2017, pp. 21–56, 2010.
[46] J. T. Barbey, J. C. Pezzullo, and S. L. Soignet, “Effect of arsenic trioxide on QT interval in patients with advanced malignancies,” J. Clin. Oncol., vol. 21, no. 19, pp. 3609–3615, 2003.
[47] D. Gaynor and D. M. Griffith, “The prevalence of metal-based drugs as therapeutic or diagnostic agents: beyond platinum,” Dalt. Trans., vol. 41, no. 43, p. 13239, 2012.
[48] J. M. Watts and M. S. Tallman, “Acute promyelocytic leukaemia: What is the new standard of care?,” Blood Rev., vol. 28, no. 5, pp. 205–212, 2014.
[49] A. T. Look, “Arsenic and Apoptosis in the Treatment of Acute Promyelocytic Leukemia,” J. Natl. Cancer Inst., vol. 90, no. 2, pp. 86–88, 1998.
[50] H. Chen, R. C. MacDonald, S. Li, N. L. Krett, S. T. Rosen, and T. V. O’Halloran, “Lipid encapsulation of arsenic trioxide attenuates cytotoxicity and allows for controlled anticancer drug release,” J. Am. Chem. Soc., vol. 128, no. 41, pp. 13348–13349, 2006.
[51] H. Kantarjian, E. Estey, D. Thomas, S. O. Brien, and J. Cortes, “Use of Arsenic Trioxide ( As 2 O 3 ) in the Treatment of Patients with Acute Promyelocytic Leukemia,” pp. 2218–2224, 2003.
[52] Z.-X. Shen et al., “All-trans retinoic acid/As2O3 combination yields a high quality remission and survival in newly diagnosed acute promyelocytic leukemia.,” Proc. Natl. Acad. Sci. U. S. A., vol. 101, no. 15, pp. 5328–35, 2004.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Medical"
The word Medical refers to preventing or treating injuries or illnesses, relating to the study or practice of medicine. Medical care involves caring for a patient and helping them through their journey to recovery.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




