Health Disparities in Prostate Cancer: An Immunobiological Perspective
Info: 8800 words (35 pages) Dissertation
Published: 5th Jan 2021
Tagged: Health and Social CareMedicine
Abstract
Prostate cancer (PCa) is the most commonly diagnosed male malignancy and the second leading cause of cancer-related death in men older than 40 years in the United States. Higher incidence of prostate cancer occurs in African-American (AA) men than European-American (EA). The current investigations to identify potential preventive factors or targets for the therapeutics intervention on the incidence of PCa related health disparities in AA men suggest that there is a difference in the genetic makeup of these populations. These genetic changes may result in environmentally induced variations such as diet and lifestyle, which are quite different in these populations. Further studies suggest that men who immigrated from Eastern to Western countries demonstrated a higher risk of prostate cancer than the men of native countries. However, the number of immigrants developing prostate cancer incidence still does not match native black/white men, therefore genetic factors also play a crucial role in the development of prostate cancer, which is also supported by familiar studies. Altered genetic polymorphisms are also associated with PCa progression. Androgens and its receptors (AR) play a major role in the development and progression of PCa. Indeed, several differences, including genetic makeup, genetic mutations, environmentally induced variation, lifestyle, and diet etc., have been reported in malignant prostate tissue from patients with a diverse background. Here, we attempt to provide an immunobiological perspective on PCa racial disparities by collecting available information on the associated factors and discussing their importance in disproportionate incidence and clinical outcomes.
Introduction
Prostate cancer (PCa) is the most commonly diagnosed male malignancy and second leading cause of cancer-related death in men older than 40 years in the United State [1]. However, there is a significant disparity between the incidence and mortality of this disease among different race/ethnicity and countries. The prostate cancer (PCa) incidence is declining, but the overall prostate cancer-related mortality continues to rise in African-American (AA) men [1]. Studies suggest that the AA population is more susceptible to PCa than European-Americans (EA); thus, the AA population has a higher risk of PCa than EA population [2, 3]. Further evidences suggest that there may be multiple reasons for the disparity in mortality rate between AA and EA men for PCa than any other malignancy [2]. In brief, AA men are often diagnosed with more advanced and aggressive PCa when compared to any other racial/ethnic groups. The rate of mortality among these populations can partially be addressed by the environmental factors, diet, genetic makeup, and lifestyle etc. These factors have been distributed differentially in a different group of populations. These factors may be associated with prostate carcinogenesis through the genetic mutations, which are deleted/edited time to time at various frequencies between these populations [4].
So far, several studies have carried out to investigate the molecular and biological mechanism(s) of PCa racial disparities; however, precise underlying mechanism(s)/causes remain unclear. It is commonly believed that environmental factors, health care, genetic makeup, cultural and socioeconomic factor, diet and lifestyle, and awareness and motivation for prostate cancer screening might be the responsible reasons for PCa disparity among these populations [5]. However, genetic and molecular levels could be more crucial than other thought for racial disparities in PCa incidence and outcome [2]. In this revival, we will focus on the immunobiological perspective of PCa racial disparities by collecting available information on the associated factors and discussing their importance in excessive incidence and clinical outcomes.
Prostate cancer health disparities and population groups
Health disparity is a difference in the incidence, prevalence, mortality, and burden of cancer and cancer-related adverse effects that exist among a specific group of the population(s). Thus, the explanation of disparity is not limited to cancer as the disease but also includes both the conditions that develop in cancer and the effect of these conditions on the quality of life and mortality. The conditions that contribute to the cancer health disparities have an extreme impact on the underserved populations. Studies on how disparities impact a patient’s life and mortality have demonstrated that underserved populations showed poor survival after a cancer diagnosis [6-8]. The applied research on prostate cancer health disparities suggested that clinical practice is contributing to improving health outcomes of cancer patients.
Moreover, the specific group of the population suffering from cancer health disparities can be categorized based on the genetic makeup, age, socioeconomic status, geographic location, gender, and race/ethnicity. The difference in the incidence and the mortality determined in male for PCa racial disparity can be obtained from Global cancer incidence data base. Racial disparities among different race/ethnicity populations may be different; however, in specific group of population for example AA population group, has the highest overall cancer burden in the USA and an increased mortality rate than EA population group [9, 10]. Furthermore, the incidence and mortality trends observed in Hispanic and Latino populations in the USA resemble those observed in Latin American nations (Figure 1).
In the United States, based on race/ethnicity population can be defined self-reported European-American (EA), African-American (AA), Hispanic-Latino (HL), Native-European (NE) and Native-American (NA). Surveillance, epidemiology and end results (SEER) program classified these populations. However, classification of this population in five race/ethnic groups is more compromise than an ideal solution. To address the concern of health disparity, we should also incorporate social description when characterizing minority population [11]. Self-identification of race/ethnicity can be acceptable in some area; however, it may be a poor way to identify the ancestry in other population group and area [12-14]. Moreover, the hidden population may also lead to false positive discoveries in the studies associated with genetic variations, therefore, it has been suggested that to determine cancer health disparity, one should include genetic factors such genetic history in the place of using self-identified or self-determined race/ethnicity [12-14]. Thus, cancer health disparity research prone artifact and ancestry marker has been developed to correct the potential for cofounder and can readily be used to characterize the ancestry such AA, EA, and NA (Figure 1) [14-16].
Please insert figure 1 here
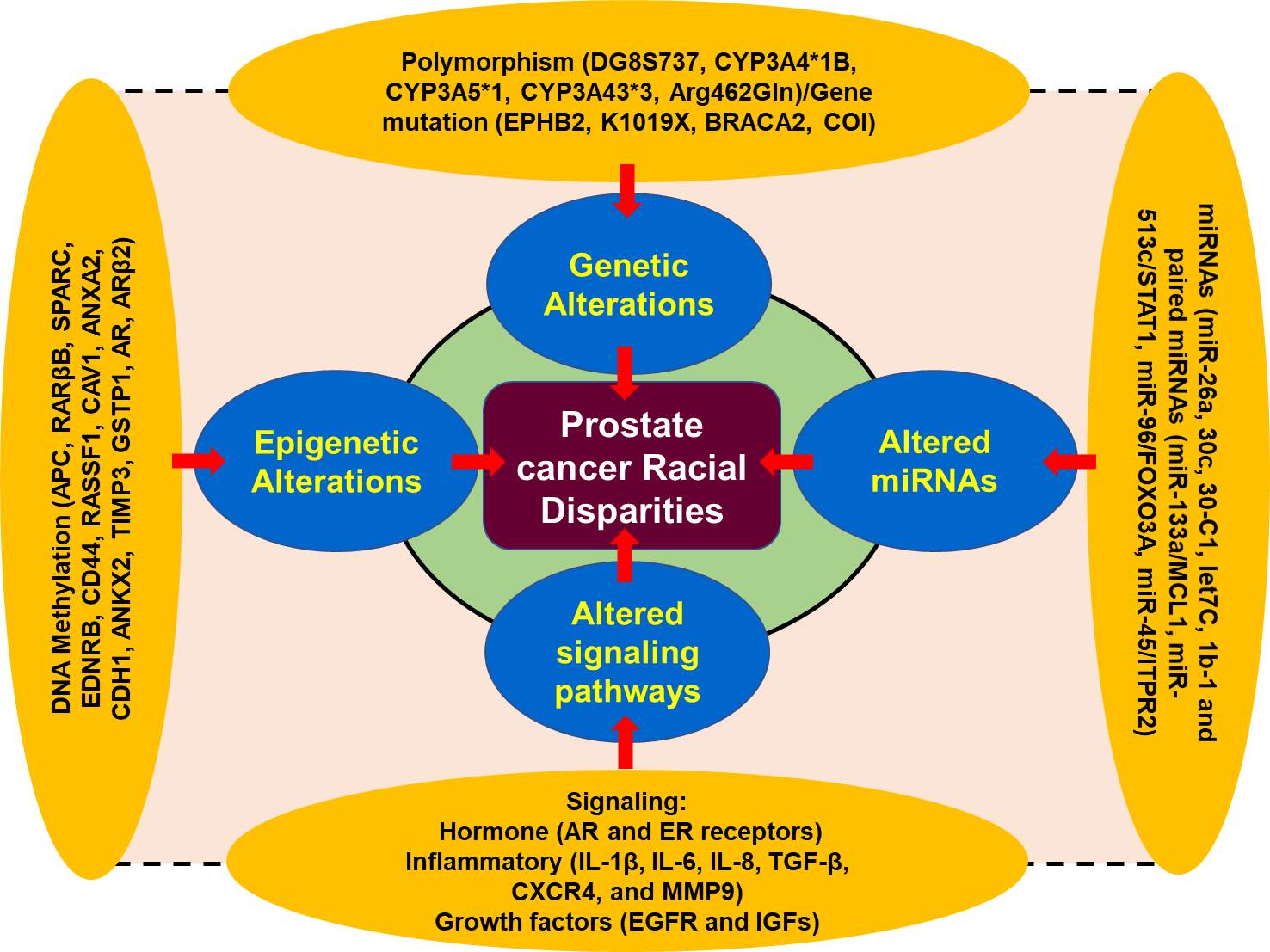
Figure 1. A schematic presentation of the factors associated with prostate cancer disparity[2] [17-32].
Genes Involve in PCa tumor biology in different race
Recent advances in molecular biology revealed the fact that the genetic makeup of different population group is different in prostate cancer disparity for example AA men are more susceptible for PCa than EA men. There are several race/ethnic genetic abnormalities which have been found associated with high risk of prostate cancer occurrence. Studies demonstrated that genetic alteration, and their possible association with prostate cancer disparities are reported in the chromosomal region of 8q24 and the genes CYP3A4 and EphB2 [33-36].
Genetic Polymorphism
Genetic polymorphism, one of the most important factor for the disease prostate cancer susceptibility and therapeutic outcomes. The CYP3A4 gene, belonging to cytochrome p450 family, is well-known to play a critical role in testosterone metabolism and involved in prostate cancer occurrence and aggressiveness [33-36]. CYP3A4 allele variation among different racial groups are significant in AA men when compared to EA men, therefore, AA men exhibited higher frequencies of the allele as compared to the EA men [2]. On the other hand, polymorphism of CYP3A4*1B allele is inversely proportional to the development of cancer in EA men. Furthermore, single nucleotide polymorphism (SNPs) in the 5’ terminal of the CYP3A4 gene which received adenine to guanine. CYP3A4*1B, and alternative as CYP3A4-V, CYP3A4-392A>G, have been found associated with a high clinical grade of prostate cancer in men [11]. Studies suggest that the frequency of G alleles was differentially distributed among the population of different race/ethnicity such as AA men [12,13]. Furthermore, distribution of AA, AG, and GG alleles among the population is closely associated with race/ethnicity, with 92% EA men having AA allele while AA men have 17% [33]. The GG homozygous and AG heterozygous allele proportion are 1% and 7% in EA men vs 43% and 39% among AA men [33, 37]. Further analysis revealed that there was a significant association between the CYP3A4*1B genotype and reoccurrence of PCa, and AA men with CYP3A4*1B genotype are more sensitive compared to EA men [33, 38].
Furthermore, AA and EA men exhibited a statistically significant difference in the frequencies of alleles CYP3A5 and CYP3A43; however, CYP3A5*1 and CYP3A43*3 a1lele frequencies may be higher in AA men. Thus, occurrence of the genotypes CYP3A4*1B-CYP3A5*1 was directly related to the prostate cancer progression in AA men. A pairwise interaction between the CYP3A4*1B and CYP3A43*3 suggested protective against PCa progression.
Furthermore, to deepen our understanding regarding prostate cancer health disparity we need to understand the role of androgen receptor (AR) signaling in prostate cancer pathophysiology. AR consists two polymorphic repeats of high frequency, CAG (19-20 in AA men) and GGN (21-22 in EA men). Studies demonstrated that shorter lenth of CAG repeats responsible for PCa disparity in AA men as compared to EA men. [7, 21] Moreover, allelic variations in chromosome 8q24 play critical role in PCa health disparity and has been found associated with high risk of several common epithelial cancers including prostate cancer that contributes to the heredity of PCa by altering allele-8 of the microsatellite DG8S737 [39]. These associations between 8q24 variants lead to the susceptibility of PCa in AA and EA men [40]. Genome-wide studies revealed that 16 loci on the chromosomal band 8q24 associated with high risk of PCa in various populations worldwide [41-43]. Additionally, enhancer elements located on the 8q24 regions associated with increase susceptibility to PCa have been reported to increase MYC promoter activity [44]. Among these genetic variations, the SNPs on 8q24 (rs4242382-A, rs6981122, rs7000448, rs16901896) were found significantly associated with the high risk of PCa in AA men than EA men (Figure 1) [44].
Gene mutation
In addition to gene polymorphism, gene mutation also plays a significant role in contributing to the racial disparity in PCa among the races. Studies show that EPHB2 gene regulates the expression of EphB2 receptor tyrosine kinase, which reported as tumor suppressor genes [45-47]. Mutation in this gene associated with high risk of prostate cancer in AA men than EA men. In AA men, the frequency of a germ line EphB2 nonsense variant (3055A→T; K1019X) was associated familial ancestry of PCa [45-47]. Further, the analysis revealed that EphB2 locus was found associated with higher risk of prostate cancer in AA men compared to EA men. Thus, mutation in EphB2 receptor tyrosine kinase suggest that AA population are more prone for PCa disparities than EA population. EphB2 SNPs showed statistically significant association with PCa risk, among the races and the most significant coding for synonymous coding SNP, referred to as TGen-624. Two SNPs showed significant associations towards a protective effect, those were rs10465543 and rs12090415 respectively. Furthermore, haplotype analysis revealed that low level of linkage equilibrium within the regions, with 2 blocks being associated with aggressive PCa risk [46, 47]. The high rate of mutation present in AA men indicates the importance of racial disparity in PCa, which clearly differentiate AA population from EA population (Figure 1).
MicroRNAs
MicroRNAs are small, endogenous, 18-25 nucleotides long non-coding molecule that play critical role in the regulation of gene expression. They block mRNA function either by inhibition of translation to the complementary region in the 3’ untranslated regions (UTR) or degradation of the target mRNA [2]. Studies suggest that miRNAs are playing a critical role in cancer related racial disparities among different races/ethnicity by promoting tumor development, and therapy resistance [29, 48, 49]. In several tumors including PCa, altered expression of microRNAs results in the tumor growth and progression [29, 48, 49]. For example, miR-26a, miR30c, miR30-C1, miRlet7C, miR1b-1 and paired miRNAs (miR-133a/MCL1, miR-513c/STAT1, miR-96/FOXO3A, miR-45/ITPR2 are associated with advance risk of PCa [27, 28]. The expression of miR-26a was found higher in AA men with PCa as compared to EA men. Interestingly, the expression of miR-26a in AA men was found directly proportional to the aggressiveness of PCa when compared to the EA men. MiR-30c and let7c also showed differential expression in difference races/ethnicity. Additionally, in AA men, 18 out of 22 miRNAs signature have been found associated with high risk of PCa disparity in this population [50]. Bioinformatic based analysis of suggested that miRNA-mRNA pairs can detect the activation of EGFR-PI3K-AKT signaling in PCa racial disparity between AA and EA men (Figure 1).
Epigenetic factors in racial disparity
Changes in organisms induced by modifications of gene expression rather than alteration in the genetic makeup, called epigenetics. The post translational modification of histone proteins related to DNA that changes gene expression along with the expression of gene variants are associated with PCa epigenetics. A broad range of epigenetics markers such as methylation, acetylation, phosphorylation, sumoylation and ubiquitination are playing critical and complicated role in regulating the expression of genes in many cancers including PCa [51, 52]. The most frequent epigenetic modification in prostate cancer is DNA hypermethylation of CpG islands (addition of methyl group to cytosine-guanine dinucleotide). DNA hypermethylation can altered gene function by inhibiting transcriptional factors/activator to the target sites [53]. Several studies have demonstrated that the frequency of DNA hypermethylation in normal as well as malignant cells are race/ethnicity specific, which may greatly contribute to the PCa racial disparities [32, 54, 55]. For example, APC, RARβB, SPARC, EDNRB, CD44, RASSF1, CAV1, ANXA2, CDH1, ANKX2, TIMP3, GSTP1, AR, ARβ2) are found hyper methylated in AA men as compared to the EA men. Therefore, existing data suggest that epigenetic factors are also playing a significant role in racial disparity of PCa among AA and EA populations and may be potential targets for cancer therapy (Figure 1).
Tumor biology contributing factors in PCa disparity
Studies suggest that the undeserved population/minority population remain discriminated in clinical trial as compared to the deserve population (white population) or population of critical importance race/ethnicity. Therefore, it is hard to identify the efficacy of cancer therapies among the populations if the population is not diverse, which may be the same or similar for AA, EA, Hispanic/Latino, and Native Americans patients [4, 56, 57]. Currently, the mortality rate of PCa patients in the USA seems to be highly improved for each race/ethnicity; however, survival groups among individual are persisting [4]. Studies suggest that AA men tend to be more aggressive at PCa diagnosis than they are in EA men. This observation gained further importance in understanding the biology of these tumors. Study suggests that before diagnosis the probability of the occurrence of cancer between the race/ethnicity were observed similar at the subclinical stage [33]. Among men with different races/ethnicity who have detectable PCa, AA men seemed to have faster growing and more aggressive tumors than EA men [58]. Additional investigation will explore whether mechanisms related to an increase proliferation or inhibition of apoptosis, promotes the enhanced growth of tumors in AA men. Epidermal growth factor receptor (EGFR) signaling is anti-apoptotic, which contributes to the activation of other signaling pathways such as MAPK, PI3K/Akt and nuclear factor ksppa-B (NK-kB. Therefore, it is plausible to target the oncogenic molecule, which is critically important for the occurrence of androgen-independent malfunctions. Androgen-independent pathways have been widely studies in relation to the development of advance PCa [59]. These findings suggest that receptor-mediated signaling is more prevalence in AA men that inhibits apoptosis and promotes cell proliferation and differentiation as compared to the EA men [2, 60].
Androgen receptor pathways
Androgens are hormones that are essential for normal male sexual characteristics. Signaling of androgen hormones require intracellular hormone receptor and a transcription factor, which play an importance role in the development and progression of PCa. Emerging evidence suggest that AR signaling pathways is one of the key biological pathway related to PCa racial disparity in AA men. Androgen receptor (AR), a hormone-dependent transcription factor, belongs to the nuclear hormone receptor superfamily [61]. AR remains in the cytoplasm of stromal and secretory luminal cells when androgens are absent. To stabilize active structure and proteolytic cleavage, AR binds to cytosolic HSPs (Heat shock proteins) [62, 63]. Androgen stimulates the activation of AR that plays a critical role in sexual differentiation, development, and maintenance of maleness. Testosterone, an androgen, derived from cholesterol. Leydig cells of the testis actively participated in the synthesis of testosterone and considered primary source of testosterone. After Leydig cells, adrenal cells also synthesize testosterone to a lesser extent and plays a critical role in androgen receptor signaling [62, 63]. As a hydrophobic steroid hormone, testosterone gets reduced to 5α-dihydrotestosterone (DHT) by the action of 5α-reductase [62-65]. Binding of DHT to AR leads to the dissociation of HSPs resulting in homodimerization, phosphorylation, and nuclear translocation of AR [66-69]. In the nucleus, AR binds to the androgen responsive elements (AREs) of the target genes resulting in activation of transcription initiation that leads to cell proliferation, differentiation, and secretion. Transcription initiation complex includes many co-regulatory proteins such as steroid receptor coactivator (SRC-1), p160, androgen receptor activator (ARA), and CREB binding protein (CBP) (Figure 2) [70]. Thus, evidence suggests the role of AR signaling in the development and progression of PCa racial disparity in AA men relative to EA men. Considering that AA men have higher risk of PCa than EA men through activation of this pathway.
PI3K/Akt/mTOR pathway
In advance prostate cancer, alteration of components of the PI3K/Akt/mTOR signaling pathway including a mutation in tumor suppressor gene such as PTEN is more frequent and correlated with high Gleason score and stage, chemotherapy resistance, and characteristic of advanced prostate cancer [23, 71]. These alterations result in activation of PI3K/Akt/mTOR signaling and accumulation of abnormalities such as less expression of inhibitory phosphate like PTEN. PTEN is the negative regulator of PI3K/Akt/mTOR survival pathways. Advanced prostate cancer has frequently been shown a high level of activated Akt [23, 72]. Akt-mediated receptors signaling includes insulin receptor, epidermal growth factor receptor (EGFR), insulin-like growth factor receptor (IGFR), and interleukin-6 receptor, platelets derived growth factor receptor (PDGFR) and it’s likely to function as a cellular sensor for nutrient and growth signals [73, 74]. Akt pathway regulates cell growth, differentiation, and apoptosis and angiogenesis through the mTOR (mammalian target of rapamycin) pathway and facilitated translation signals of c-myc, cyclin-D, and vascular endothelial growth factor [72]. Inhibition of PTEN and mTOR activation can suppress the growth of PTEN-/- prostate cancer xenograft in mice by restringing chemotherapeutic sensitivity (Figure 2) [23], [75, 76].
EGFR and PDGFR pathways
Several growth factors are known to promote tumor growth and differentiation, have been identified as potential causes for PCa racial disparities. Available information on the role of epidermal growth factor receptor (EGFR) signaling in PCa carcinogenesis suggest that EGFR signaling pathways may be promising target for PCa racial disparity in different races [60] EGFR belongs to tyrosine kinase family, mutations in target tyrosine kinase such as EGFR, Bcr-Abl, and c-Kit resulted in the development of this tumor, which plays an important role in the pathogenesis of these tumors. In prostate cancer, EGFR signaling is critical and involved in androgen-independent progression of PCa. Inhibitors of EGFR can block the growth of both androgen-dependent and androgen-independent signaling pathways in PCa xenograft; however, trials on tyrosine kinase inhibitors are still disappointing.
Furthermore, EGFR is highly expressed in 50-80% of the prostate cancer cells, which was commonly found in AA men with PCa as compared to EA men. Preclinical findings suggest that EGFR expression is positively correlated with Gleason score and androgen-independent progression of prostate cancer [23, 77]. Phase-II studies on EGFR inhibitor gefitinib demonstrated minimal cytotoxicity while no PSA response [23, 78]. Moreover, in prostate cancer, the resistance to gefitinib may be associated with the hyper-activation of PI3K/Akt pathway.
Prostate cancer cells also express a high level of platelet-derived growth factor receptors (PDGFR), which signaling converges with the PI3K/Akt pathway that cooperates in prostate cancer progression [79, 80]. Targeting PDGFR pathway using single therapeutic regimen such as imatinib may not be too effective therefore combination therapy is being used to target the PDGFR pathway[81-85].
Another potential target in the same family is the HER2/neu tyrosine kinase, which is found upregulated in an androgen-dependent manner leading to prostate cancer growth and survival [86, 87]. Targeting Her2/neu tyrosine kinase using a monoclonal antibody trastuzumab showed promising outcomes with less cytotoxicity (Figure 2).
IGF pathways
The insulin-like growth factor type-1 (IGF-1) and its ligands may also play a critical role in prostate cancer carcinogenesis through several mechanisms such as mitogenesis, anti-apoptosis, and cellular transformation. Studies suggest that high expression of IGF-1 and reduced expression of insulin-like growth factor binding protein-3 (IGFBP-3) has been found associated with high risk of prostate cancer [88, 89]. Additionally, IGF-1R expression was observed as higher in a prostate cancer that leads to the induction of cancer cell proliferation and resistance to androgen ablation therapy [88-91]. IGF pathway can be targeted using monoclonal antibodies (such as IMC-A12 or cixutumumab and CP-751,871 or figitumumab, fully human IgG1 and IgG2 antibodies that specifically target to IGF pathway) that bind to the extracellular domain of the transmembrane receptor [90, 91]. These molecules can inhibit the growth of both androgen-dependent and androgen-independent tumors (Figure 2).
Cytokine signaling
Tumor microenvironment consist of several types of immune cells, adipocyte, blood vessels that secreted soluble and insoluble factors such as cytokines and growth factors. Cytokines such as Interleukin-6 (IL-6), a pleiotropic molecule, involved in the regulation of several cellular processes including cell cycle, apoptosis, migration, and angiogenesis. Studies suggest that the level of IL-6 in tissues and sera was observed higher in patients with PCa [92, 93]. Therefore, it is important to know the level of signal transducer and activator of transcription (STAT), mitogen-activated kinases (MAPK), and phosphatidylinositol 3kinase (PI3K), which was observed high during IL-6 treatment. However, there is no doubt that PI3K and MAPK pathways are involved in the carcinogenesis, thus, the role of STAT3, which is crucial for IL-6 downstream signaling in normal as well as cancerous prostate tissue, need to be explored. Moreover, STAT3 is an important molecule that regulates cellular functions in prostate cancer and several attempts have been done to explore its role in the prostate carcinogenesis. Several therapeutic approaches including an anti-sense oligonucleotide, anti-IL-6 or anti-IL-6R, and CpG-STAT3-specific siRNA have been designed, but the results are still disappointing [94-96]. Even though IL-6 therapy did not show therapeutic or survival benefits in patients with metastasis PCa. Moreover, anti-IL-6 agents such as siltuximab or JAK/STAT3 inhibitors were not only ineffective but also increased the expression of proliferation marker in PCa (Figure 2) [97, 98]. Based on these information, we can assume that inappropriate tumor microenvironment may also contribute to increasing incidence and unfavorable outcomes in AA men.
Please insert figure 2 here.
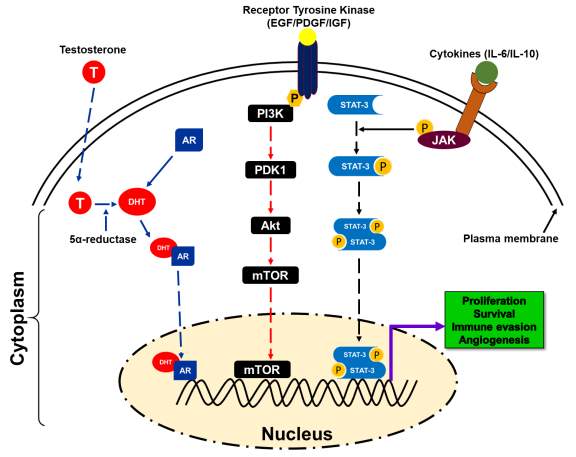
Figure 2. A schematic presentation of different pathways involved in prostate cancer disparity [33-90].
- Tumor Antigens
- Based on serological analysis and T cell that recognized antigens including tumor antigen, has exposed new area for designing and developing new therapeutic regimens to treat various kind of cancers including prostate cancer [99-102]. Thus, tumor antigens come in two flavors those are as follows:
- Defined antigens
- Undefined antigens
Defined Antigens
There may be several useful characteristics when defined antigens are used for therapeutic intervention, which are associated with the type of antigens, interaction, size, and immune responses. These properties suggest that one should target the specific type of HLA molecule. This approach has been most widely used in clinical settings either single or in combinations. Combination therapy is an emerging area for further research; therefore, the combination of two or more antigens demonstrated significant outcome in this area and answered several concerns regarding how antigens escape immune attacks. Based on these properties defined, antigens can be categories into the following categories (Table 1).
Table 1: Class of defined antigen for immunotherapy [91-119].
| Antigen | examples |
| Universal antigen | WT1, TERT,Telomerase and survivin |
| Cancer-testis antigens | SUV39H2, MAGE, BAGE, GAGE, TMEM31, NY-ESO-1 [103-106] |
| Unique antigens | β-catenin, P53, ras, CDK4, CDC27, and α-actinin-4 [107] |
| Differentiation antigens | Tyrosinease, TRPI/gp75, TRP2, gp100, Melan-A/MARTI, gangliosides, PSMA [99, 108] |
| Overexpressed antigens | HER2, WT1, EphA3, EGFR, CD20[109-114] |
Cancer-testis antigens
The group of proteins are normally expressed in the testis of adult male; however, these proteins are expressed aberrantly during tumor progression in many types of cancer. Additionally, cancer testis antigens are expressed on the variety of cancer cells. MAGE-1, a testis antigen, induces CD8+ cytotoxic T cell response. Different antigens from similar families have been detected as MAGE, BAGE, and GAGE) [100-102, 115-117]. The best studied cancer testis antigens belong to the MAGE-A family and the NY-ESO-1 which is found in the germ cell and expresses class-I and class-II restricted epitopes [100-102, 115-117]. In the normal tissue, restricted expression of these antigens results in high temperature and autoimmune dysfunctions; however, the possibility of the latter one is less than fever/high temperature (Table 1).
Unique antigens
This class of antigens show unique type of properties which are defined by T cells epitopes and exhibit great potential of immunogenicity. Some of these antigens derived from the mutation of genes may exhibit compatible biological properties for example, antigens that are less susceptible to immunoselection may maintain advance tumor. Therefore, immunogenicity and constitutive expression of tumor antigens could be a novel approach to design and develop new immunotherapies in this field. Additionally, mutation in proto-oncogene and tumor suppressor gene results in the generation of active oncogene such as CDK4, p53, ras, and b-raf [118]. The chance of mutation occurrence in proto-oncogene such as ras/raf is higher than others. Protein product after mutation in these genes induces activation of the innate as well cell mediated arm of the immune system [119-121]. The T cell immune response requires a unique type of epitope expression that targets HLA-A*24 that signaling leads to the activation of cytotoxic T cell mediated killing of the tumor cells [122]. Furthermore, CD8+ cytotoxic T cells can recognize if CDK4 acquired a substitution mutation in it [123]. However, a mutation in CDC27 results in altered expression of protein trafficking into an endosomal compartment that further leads to the presentation of MHC-II epitope and recognition by CD4+ T cells [124]. Moreover, CD8+ T cells can recognize a mutated peptide of α-actinin4 (about 10 amino acid long) [124]. Accumulation of α-actinin4 results in actin bundling in the cytoplasm which enhances cell motility and contributes to the metastasis (Table 1) [125].
Differentiation antigens
The tissue/cell specific biomolecules present on the tumor as well as normal cell/tissue are called differentiation antigens. Differentiation antigens are the best examples to study tumor targeted immunotherapy in relation to restricted expression of HLA types during tumor progression. For example, tyrosinase can be considered as rate limiting enzyme for melanin biosynthesis. It stabilizes the activity of tyrosinase by regulating the expression of tyrosine related protein-1 (TRP-1), studies suggests that in melanoma patients, serum IgG that immunoprecipitant TRP-1 can be recognized by the immune system [126]. Furthermore, the sequences of tyrosinase exhibits epitopes variations such as TRP-1 and TRP-2; therefore, it can involve in different immune responsive mechanism(s) [127, 128]. Studies suggest that in mice, TRP-1 based vaccination protects the host in antibody-dependent manner by proving passive immunity [129, 130]. In addition, Melan-A could be a target antigenic protein for cytotoxic T cells; however, it needs further investigations [129, 130]. Gangliosides (GM3, GM2, GD2, and GD3) are another group of differentiation antigens in which GD3 is the representative of the related families such as MAGE, BAGE, and GAGE. However, NY-ESO-1 unrelated to other members mentioned above (Table 1).
Overexpressed antigens
Overexpressed antigens on cancers represent the attractive target to study immunotherapy. Overexpressed antigens were found upregulated on tumor cells as compared to the normal cells. For example, proto-oncogenes, which showed similar homology among the family members (HER2, shared homology with tyrosine kinase and EGFR) [131-133]. Overexpressed antigens such as glycoprotein showed poor prognosis due to the aggressiveness of the disease [99]. HER2 protein expression was commonly found upregulated on several cancers including prostate cancer. In breast cancer cells, trastuzumab, a humanized monoclonal antibody induced cytotoxicity in an antibody-dependent manner by induction of apoptosis; however, it has limitations [99]. In order to activate both arms of the immune system by T cell activation and antibody production, some variants were deleted [99]. This modification in the antibody has been found effective in generating the antitumor immune response in mice [134, 135]. Wilm’s tumor1 (WT1), and Ephrin receptor (Eph3) proteins have also been found upregulated and sensitive target for immunotherapy [136]; however, EGFR and CD20 are the examples of passive immunotherapy in colorectal cancer (Table 1) [99, 137, 138].
Undefined antigens
Undefined/unidentified antigens are present in both allogenic and autologous vaccine settings such as intact cell, cell lysate, total RNA vaccine, and heat shock proteins. The benefits of having universal antigens are: they can easily recognize by the immune system due larger size and surface. Tumor cells are known to reduced key markers on the surface to escape immune surveillance [139]. It seems plausible to target unidentified tumor antigens for cancer immunotherapy, because vaccine based on these antigens will evoke a strong immune response compared to other (Table 1).
T cells and Antigen Recognition
T cells, a group of lymphocytes produced and processed by the thymus gland and actively participate in the immune response. Activation of T cells is regulated and requires the turning-on and -off of several switches in a timely and coordinated fashion. To start with, the T cell receptor (TCR) must engage with its cognate tumor peptide antigen which, for this purpose, must be bound to major histocompatibility complex (MHC) molecule expressed on antigen presenting cells (APCs). A co-stimulatory signal must be activated and a well-characterized co-stimulatory pathway includes CD28/B7 superfamily. The CD28 receptor is constitutively expressed on the surface of T cells, and it interacts with the B7 ligands (B7-1 [CD80], B7-2 [CD86]) on professional APCs. Simultaneously, to prevent hyperactivation of T cells, cytotoxic T lymphocytes associated protein 4 (CTLA-4) and another member of the CD28 family also interact with a B7 family of ligands to inhibit hyperactivation of T cells (Figure 3).
Please insert figure 3 here
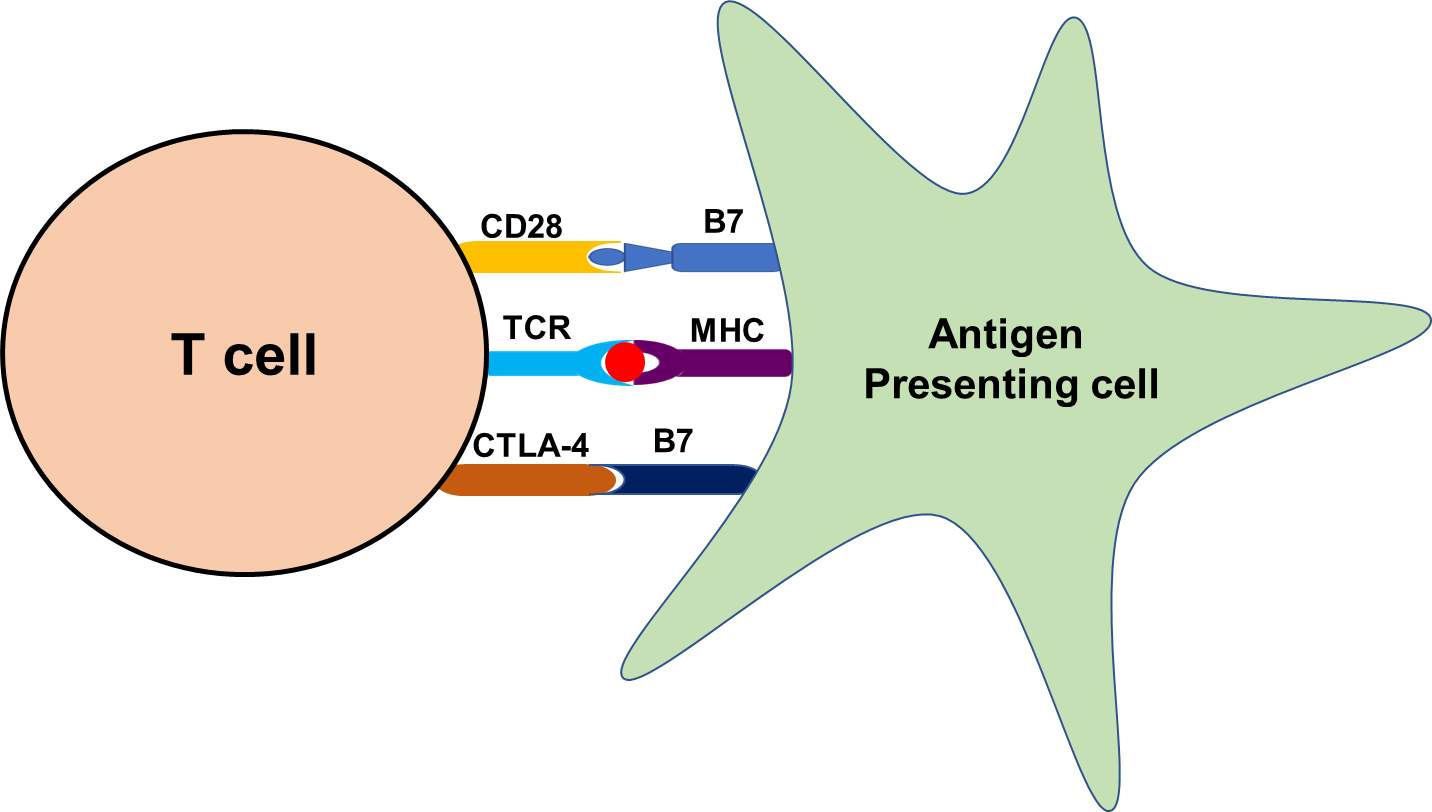
Figure 3. Representative flow diagram of the mechanism of antigen presentation through antigen presenting cell and recognition by T cells.
Role of Natural Killer T cells in tumor Immunity
NKT cells play a crucial role in the immune system, as they share common characteristics between adoptive and innate immune system. NKT cells have TCRs and antigen specificity like conventional T cells which recognize lipid antigen more potently than peptide antigen. These cells exhibit limited but rapid response characteristics of the innate immune system. Mucosal-associated invariant T (MAIT) cells and γδ-T cells can perform such specialized function. Like NKT cells, MAIT cells express TCR using Vα19jα33 chain in mice and Vα7.2jα33 in human and play a regulatory role [140, 141]. MAIT cells depend on gut flora and did not observe in germ-free mice. Some of the γδ-T cells express NK-like markers and showed unique characteristics [142-144]. Previously these cells were thought as NKT cells but are recognized as distinct T cells subsets.
Furthermore, NKT cells are the rapid responders of the innate immune system when activated and play a crucial role in the recruitment other immune cells such as NK cells, CD4+ and CD8+ T cells of the adoptive immune system [145, 146]. Thus, these cells play a critical role in orchestrating other immune responses that come later. Studies suggest that NKT cells were considered as the potent producer of Th1 (IFN-γ) and Th2 (IL-4 and IL-13) type cytokines [147, 148]. Additionally, NK1.1 negative subsets of type I NKT cells contributed in neutrophil recruitment by producing IL-17. These cells also showed the ability to produce IL-21, which can regulate NKT by autocrine fashion [149]. Studies suggest that the presence of cytokines such as IL-4 and IFN-γ allow cells to respond quickly without the need of further activation and gene transcription [150, 151]. Early production of cytokines like IL-4 by NKT cells induce a Th2 type immune response and production of IgG; however, defective IgG production in mice was associated with the absence of CD4+ NKT cells that made IL-4 [152, 153]. Although, NKT cells may not be the only source of IL-4; however, their ability to response first and subsequent adoptive responses makes their regulatory functions more influential throughout the immune system.
Like conventional T cells, NKT cells can act as a part of the adoptive immune system by the response by recognizing and presenting lipid antigens. The ability of NKT cells to recognize self-lipid antigens may lead to the profound impact of autoimmune diseases while the ability to recognize bacterial lipids, gives the adoptive T cell immune response by detecting their lipid content as well as proteins [154-156]. Thus, NKT cells serve as regulatory cells and potentially effector cells in context to autoimmune disease and allergy to infectious disease and cancer (Figure 4).
Please insert figure 4 here
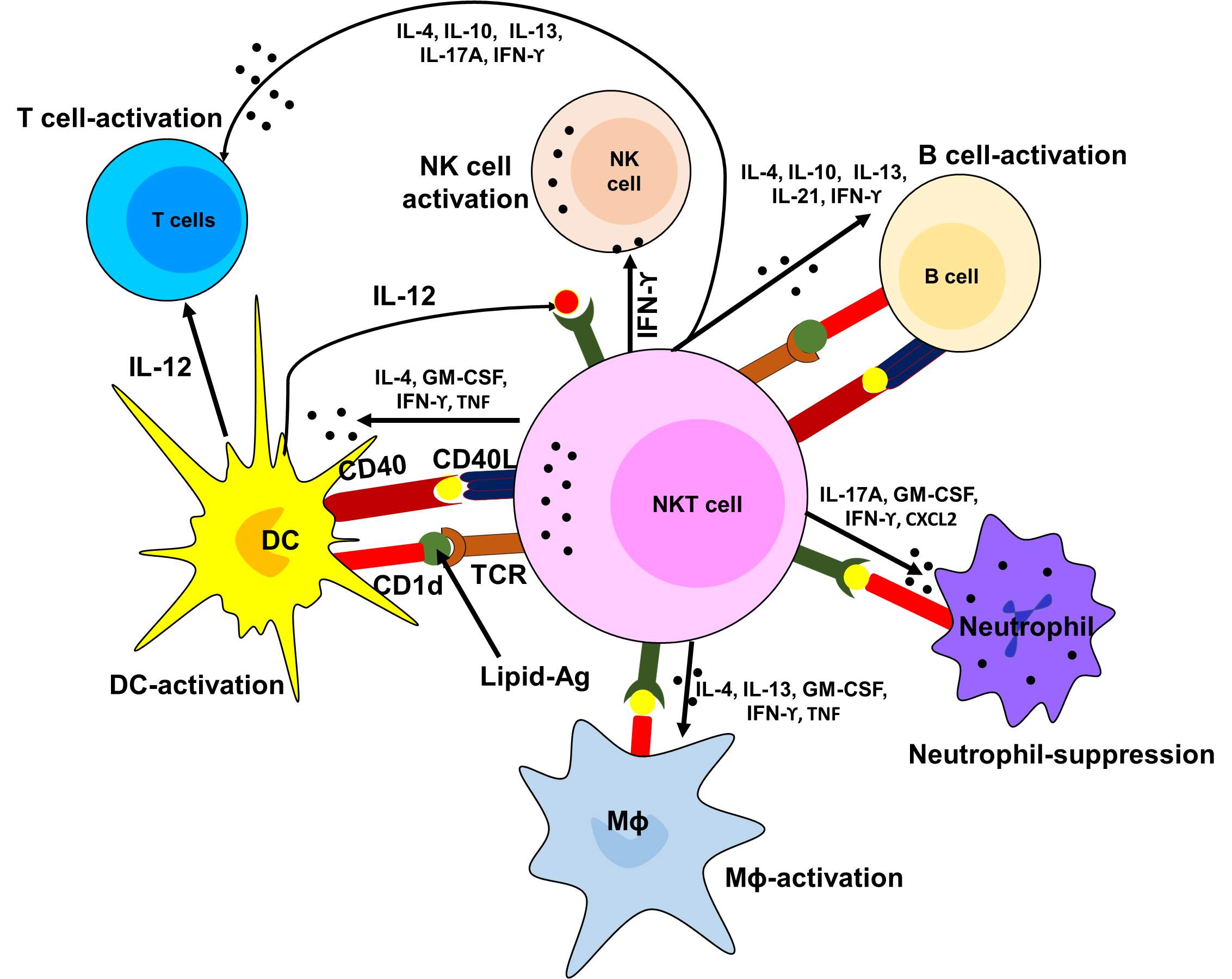
Figure 4. Diagrammatic presentation of the role of NKT cells in the activation of other immune cells.
Immunomodulatory Molecules of the Immune System
Cytokines act as communication bridge among the cells of both adoptive and innate immune system. They are primary immune modulator and communicator in response to infection and associated immune challenges and are involved in several biological processes. As a part of the signaling network, cytokines can stimulate and modulates immune system function and induce their own synthesis and the synthesis of other cytokines. Cytokines are soluble in nature; however, some remain cell-bound. Cytokines can be divided into pro-inflammatory (such as IL-1β, IL-6, and TNF-α) and anti-inflammatory cytokines (such as IL-10, TGF-β) [157, 158]. Chemokines are the subpopulation of cytokines that recruit other cells through chemical stimulation [159, 160]. Cells attracted to the chemokines follow an increased strength of signal towards the source of chemokines such as infected or damaged cells. As signaling molecules, cytokines follow receptor-mediated cell signaling and exhibit their impact by stimulating intracellular signaling cascades. Moreover, based on the distance of source and target, cells, cytokines can further be classified [159, 160]. Cytokines passed through the blood before reaching the target cells, called endocrine. Cytokines that act near the secreting cells are called paracrine. The autocrine action is when secreting cells receive by its own receptors. Based on their receptor structure cytokine and their functions can be studied as follow;
Please insert table2 of cytokine types
Table 2. Cytokines and their role [139-151].
| Cytokine | Source | Target | Functions |
| IL-1 | Mφ, Monocytes, epi- and endothelial cells, fibroblasts, astrocytes | B & T cells, endothelial cells, Hypothalamus, and liver | Co-stimulation, activation, inflammation, fiver, and acute phase reactant |
| IL-2 | NK & T cells | Monocytes, NK, B & T cells | Cell growth/activation |
| IL-3 | T cells | Bone marrow progenitor cells | Cell growth and differential |
| IL-4 | T cells | B & T cells | Th2 differentiation, cell growth/activation, IgE isotyping switching |
| IL-5 | T cells | B cells, eosinophils | Cell growth/activation |
| IL-6 | Mφ, T cells, Fibroblasts | B & T cells, Liver | Co-stimulation, cell growth/activation, acute phase reactants |
| IL-7 | Fibroblasts, Bone marrow stromal cells | Immature lymphoid progenitors | T cell survival, proliferation, homeostasis, B cells development |
| IL-8 | Mφ, epithelial cells, platelets | Neutrophils | Activation chemotaxis |
| IL-10 | Th2 T cells | Mφ and T cells | Block APCs, and cytokine production |
| IL-12 | Mφ and NK cells | T cells | Th1 differentiation |
| IL-15 | Monocytes | NK & T cells | Cell growth/activation, NK cell development, and block apoptosis |
| IL-18 | Mφ | NK, B & T cells | Cell growth/activation, and inflammation |
| IL-21 | NKT & CD4+ T cells | NK, B & T cells | Cell growth/activation, control allergic response and viral infection |
| IL-23 | APCs | DC, NK, & T cells | Chronic inflammation, promotes Th17 cells |
| GM-CSF | T cells, Mφ, endothelial cells, fibroblasts, and mast cells | DC, Mφ, NKT and Bone marrow progenitor cells | ↑ antigen presentation, T cells homeostasis Hematopoitic cells, and growth factor |
| IFN-α | B & T cells, Mφ, endothelial cells, fibroblasts, plasmocytoid DC and Osteoblast cells | Mφ and NK cells | Antiviral and ↑ MHC expression |
| IFN-β | Leucocytes | Anti-proliferative | |
| IFN-γ | NK, NKT & T cells | Mφ, monocyte, endothelial cells | Cell growth activation, ↑ MHC expression |
| TGF-β | T cells and Mφ | T cells | Inhibits cell growth and activation |
| TNF-α | T cells and Mφ | B & T cells, endothelial cells, Hypothalamus, and liver | Co-stimulation, activation, inflammation, fiver, and acute phase reactant |
Furthermore, B and T lymphocyte are playing a crucial role in maintaining adoptive immune response [161, 162]. Before getting differentiate into their functional types these cells also produce specific cytokine to achieve specific functions such as Th1, Th2, Treg, and Th17 [161, 162]. Proliferation and differentiation of functional type depend on the cytokine-meditated microenvironment and activation of TCR signaling. Differentiated Th1 and Th2 type effector cells determine the nature of the adoptive immune responses activated by effectors cells [161, 162]. Th1 produces IFN-γ, their signature cytokine, and IL-2, while many also produce TNF-α. The signature cytokines of Th2 cells are IL-4, IL-5, and IL-13, but these cells also secrete TNF- α, and some produce the IL-9 and modest amount of IL-2 [161, 162]. The immune regulation requires homeostasis between the activity of Th1 and Th2 types cells [163-167]. If one type of cells (Th1) becomes dominant another cell (Th2) type gets suppressed. The lower number of Th1 cells and a higher number of Th2 cells have been found associated with Autism spectrum disorder (ASD) in children suggest that ASD is an example of imbalance between Th1 and Th2 type cytokines [168, 169]. Additionally, peripheral blood mononuclear cells from children with ASD showed enhanced activation of Th1 and Th2 types cells of the adoptive immune response, with a dominant Th2 and no compensatory increase in the expression of IL-10 [170].
Single or multiple cells can produce the same type of cytokines such as Th1 cells produce IFN-γ, IL-2, and TNF-β; however, Th2 cell able to secrete IL-4, IL-5, IL-6, IL-9, and IL-10 [22]. However, more than one cells can secrete granulocyte monocyte colony stimulation factor (GM-CSF) and IFN-β [22]. The single signature cytokine can act on more than one cell type (IL-12 acts on Th1 cells; however, IL-1 acts on T and B cells, macrophage, endothelial cells, fibroblast cells, epithelial cells). Furthermore, almost all interferon act on multiple cells types. Multiple cytokines act on one cell and exhibit a similar effect, called cytokine redundancy. On the other hand, single cytokine may have multiple effects on a cell. IFN-γ induces antiviral proteins, enhance MHC-I by stimulating NK and IL-12 production, induces antiproliferative effects [22]. Enhanced expression of IFN-γ and IL-12 suggested inflammation while more TGF-β, IL-4, and IL-10 can suppress inflammation [99, 171].
HLA Class Antigen Abnormalities in tumors
Studies demonstrated that positive outcomes of clinical trials from T cell-based immunotherapy in PCa have gained great attention in the characterization of the antigen processing machinery component expression in PCa disparities since this machinery is playing a critical role in the generation and expression of the trimeric surface antigen complex (HLA-I) on tumor cells. This complex organizes interaction of tumor cells to a HLA-I antigen; tumor-infiltrating cytotoxic T lymphocytes (CTL) is recruited through several participations of associated/co-stimulatory molecules. HLA-I presents cytosolic/nuclear protein or endogenous peptides antigen in the range of 9-11 amino acids long [172, 173]. These peptide antigens passed from cytosol to endoplasmic reticulum (ER) through transporter associated with antigen processing (TAP) where they loaded on to β2-microglobulin (β-2m)-HLA-I heavy chain complex with the help of a group of chaperones such as calnexin, calreticulin, ERp57, and tapasin [174-176]. The trimeric HLA-I-peptide complex is now ready to transport from trans-Golgi to the cell surface for presentation to CD8+ CTL [177, 178].
Abnormalities in antigen processing machinery component expression have been associated with several types of malignancies [179]. These abnormalities demonstrated clinical significance since they can be associated with tumor progression and development and poor patient’s survival. Studies suggest that murine prostate cancer cells express MHC-I surface antigen; however, lack of detectable LMP2, LMP7, TAP1, and TAP2 transcription leads to tumor development [180]. Expression of IFN-γ induced this four antigen processing machinery components and enhanced MHC-I surface expression. The TAP aberration in murine metastatic PCa cell line caused by impaired transcription initiation of TAP1 [180]; however, constitutive expression and IFN-γ –induced transcription of TAP2 HLA-I and HLA-I surface antigen expression in several studies was also determined [180-182].
The Local Tumor Microenvironments
Studies suggest that difference in prostatic tumor microenvironment contributes to the health disparity associated with prostate cancer [183]. The contribution of genetic factors in view of prostate cancer incidence and mortality in AA men remains controversial. While some studies demonstrated that race/ethnicity is different in different prostate cancer microenvironment [183]. Prostate tumor microenvironment includes genetic as well as immune alterations. Genetic abnormalities include gene chromosomal aberrations such as 8q24, CYP3A4*1B, and EphB2 SNPs. However, immune alteration consists of low cytotoxic activity of NK cells, CD8+ T cells, high secretion of TGF-β by prostate tissue, which inhibits the cytotoxic function of NK cells and CD8+ T cells and induce recruitment and accumulation of Treg and Th17 lymphocytes that downregulates anti-tumor immunity [184]. Enhanced levels of TGF-β in prostate tissue associated with pathologic conditions and increase the likelihood of post-operative residual tumor in localized PCa [185]. Additionally, the concentration of TGF-β in prostate cancer metastasis is correlated with prostate tumor burden [185]. The prostate immune microenvironment is very dynamic, changing over the time, clinical states. The latter is associated with a series of phenotypic alterations leading to immunomodulation [186, 187] such as increasing TIL in a prostate bed following androgen deprivation therapy [188] or sensitization of tumor cells to T cell- mediated lysis and higher expression of PD-1 ligands (PD-L1 and PD-L2 expression) on resistant prostate cancer [189].
Conclusions
Many studies demonstrated the role of various factors influencing the racial disparity among AA and EA populations [2, 3, 7, 34, 60]. These factors include, changes in genetic makeup, altered expression of microRNAs, mutation and alteration in signaling, and epigenetic changes that can impact racial disparity in AA men. In this review, we provided an immunobiological perspective on PCa racial disparities by collecting available information on the associated factors and discussing their importance in disproportionate incidence and clinical outcomes. Emerging evidences suggest differential tumor microenvironment (highly heterogenous and suppressive) may also be one of the key factors for PCa racial disparity clinical outcomes. Thus, the composition (having various types of immune cell) of tumor microenvironment can differ from person to person because of the genetic makeup of the tumors, extent heterogenicity, host genetic as well as lifestyle, and dietary and environmental factors. Therefore, it can be assumed that tumor biological differences or more specifically, in the tumor microenvironment, are determined not only by host and tumor genetics but also by lifestyle, diet, and cultural behavior. Immunobiological factors may be the type of tumor antigen, pattern of presentation, recognition, and evoked immune response against those antigens, and most importantly the heterogenicity in tumors as well host genetics. This data provides us a wider and more specific perspective of the biological basis of PCa racial disparities and should direct our future endeavor in basic, clinical, and translational research. As the case of with any kind of research, availability of resources, any kind of funding support, and appropriate research tools are very important to make progress in PCa health disparity research. Thus, focused efforts at the level of team research, community, in clinics, and by our policy maker are needed to make race-associated PCa disparities as a thing of the past or at least make them irrelevant in clinical practice at each racial level.
Further Reading
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Medicine"
The area of Medicine focuses on the healing of patients, including diagnosing and treating them, as well as the prevention of disease. Medicine is an essential science, looking to combat health issues and improve overall well-being.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




