The Effect of Increasing Working Memory Load on Static Postural Control in Young and Old Adults
Info: 24044 words (96 pages) Dissertation
Published: 28th Feb 2022
Tagged: PsychologyPhysiology
Abstract
Background and purpose. The decline in postural control has shown to be primarily associated with aging and disease of the central nervous system. In the recent years, researchers have explored cortical changes during static, dynamic and virtual reality-based postural control tasks using various neuroimaging techniques. However, the mechanisms by which specific working memory tasks with varying working memory load influence cortical activations and postural control have not been delineated clearly. In the current study, the specific cortical and postural changes influenced by a working memory task with varying cognitive load during a no vision static balancing task will be explored in healthy young and old adults.
Design. A mixed (between- and within-group) cross-sectional descriptive design is proposed.
Methods. Twenty-four healthy young participants (Male=12, age= 18-40 years) and twenty-four healthy old participants (Male=12, age= 60-85 years) will perform a postural control task (double leg standing postural control) while responding to an n-back working memory task in the absence of vision. Behavioral responses as response time and accuracy, postural control responses as CoP sway area and sway velocity, and oxygenated hemoglobin concentrations (HbO) will provide cognitive, postural control, and neural performance measures, respectively.
Results. I expect to see decreased accuracy and increased response time in conjunction with a greater CoP sway area and sway velocity for tasks with greater working memory load. I also expect to see an increase in cortical activation in cognitive decision-making areas, represented as increased HbO levels, as a function of increased working memory load in young and old adults. Finally, I expect to see varied connectivity patterns between working memory, seated rest, and standing rest tasks for the two groups. The extent and nature of potential group differences will be explored in this study.
Conclusion. The results of the proposed study are likely to suggest that increasing working memory load influences postural and cortical changes, and could give us insight into the specific cortical areas involved in postural control and working memory tasks. Further, the results are likely to confirm a lawful relationship between working memory load, postural control, and cortical excitation. Finally, the nature of potential neural activation between the young and old groups will provide evidence about the role of aging in changes between information processing and postural control. A better and more complete understanding of this relationship has the potential to inform the development of targetted interventions involving the addition of cognitive tasks that vary cognitive load together with postural control practice for fall prevention.
Keywords
Falls, postural control, dual-tasking, working memory, cognitive load, neuroimaging functional Near-Infrared Spectroscopy, wearable sensors
PUBLIC ABSTRACT
Sushma Alphonsa
The decline in postural control has been shown to be primarily associated with aging and disease of the central nervous system. Cortical changes during static, dynamic and virtual reality tasks have been explored using various neuroimaging techniques. However, the effect of changing working memory load on cortical and postural control have not been answered clearly. In the current study, I explore the specific cortical and postural changes influenced by working memory task (n-back) with varying cognitive load during a no vision static postural control task in healthy young and old adults. I used a pseudo-randomized control block design for this study. Twenty-four healthy young participants (Males=12, age= 18-40 years) and Twenty-four healthy old participants (Males=12, age= 60-85 years) performed an n-back working memory task while performing a postural control task (double leg standing postural control) with no vision. Measures of interest are response accuracy, the CoP sway area and sway velocity for the postural control and oxygenated hemoglobin concentrations (HbO) that represent co-occurring working memory load, postural control, and neural activation respectively. I expect to see an increase in the cortical activation as increased HbO level with increasing working memory load. Finally, I expect to see a greater CoP sway area and sway velocity with increasing working memory load specifically, in old adults. By understanding the connectivity in cortical areas using functional connectivity analyses, this study will be the first to contribute the overall understanding of age-related cortical changes during posture-cognition dual-tasking. Such findings would be consistent with the selection for action theory of the posture-cognition dual-task paradigm. The execution of a goal-directed action (in our case, maintaining an upright postural control) requires two or more subsystems (in our case cognitive and auditory processing) to modify successfully such that the action is executed successfully. Failing which, one system is given priority over the other. These findings would contribute to the existing literature by showing the extent and nature of changes in cortical and postural measures when the cognitive load is modified.
CONTENTS
Click to expand Contents
LIST OF ACRONYMS AND ABBREVIATIONS ……………………….xiii
I. INTRODUCTION…………………………………………17
Purpose…………………………………………………21
Assumptions……………………………………………..24
Definitions of key terms …………………………………….25
II. LITERATURE REVIEW …………………………………..26
Postural control…………………………………………….26
Wearable sensors……………………………………….28
Dual-tasking and attention…………………………………30
Theories of attention………………………………..31
Working memory…………………………………………..32
n-back working memory paradigm…………………………..32
Neuroanatomy……………………………………………..34
Cortical Regions of Interest………………………………..34
Prefrontal Cortex…………………………………..36
Dorso-Lateral Pre-Frontal cortex………………………..37
Motor Cortex……………………………………..38
Somatosensory Association cortex………………………30
Neuroimaging tools for functional imaging…………………………38
Functional Near-Infrared Spectroscopy……………………….40
NIRS-SPM………………………………………41
Neural correlates……………………………………………41
Neural correlates of working memory…………………………41
fNIRS and working memory……………………………….42
Neural correlates of postural control………………………….42
fNIRS and postural control………………………………..44
Working memory and postural control………………………..45
III. METHODS…………………………………………… 47
Participants ……………………………………………..48
Procedures ………………………………………………51
Pre-assessments ……………………………………….51
n-back procedure ………………………………………52
n-back stimuli …………………………………………54
Practice ……………………………………………..54
fNIRS capping and wearable sensors setup …………………….55
Experimental protocol …………………………………..57
Head measurement ……………………………………..58
Measurement variables ……………………………………..59
Independent variables ……………………………………59
Dependent variables …………………………………….60
Data analyses …………………………………………….60
Behavioral data ………………………………………..61
Postural data ………………………………………….62
fNIRS data …………………………………………..62
Registration ……………………………………62
fNIRS data processing …………………………….63
Statistical analyses ………………………………………….64
Descriptive statistics …………………………………….64
Inferential statistics …………………………………….65
Limitations……………………………………………….69
REFERENCES ………………………………………………..71
APPENDICES …………………………………………………94
IRB consent form …………………………………………..94
Medical History Questionnaire …………………………………97
Montreal Cognitive Assessment (MoCA) ………………………….99
Edinburgh Handedness Form ………………………………….100
Physical Activity Readiness Questionnaire (PAR-Q) …………………101
TOMAL-2: Letters Forward Subtest……………………………..102
Participant data sheet ……………………………………….103
LIST OF TABLES
3.1 Physical characteristics of all participants………………………52
LIST OF FIGURES
2.1 Revised neuroanatomical model for working memory……………….32
2.2 Left Frontal Cortex……………………………………….35
2.3 Left Pre-Frontal Cortex ……………………………………36
2.4 Left Dorsolateral Pre-Frontal Cortex …………………………..37
2.5 Left Motor Cortex………………………………………..37
2.6 Left Somatosensory Association Cortex ………………………..38
2.7 fNIRS principle and Probe set-up……………………………..41
3.1 Experimental design flowchart……………………………….47
3.2 Power analysis for postural control…………………………….49
3.3 Power analysis for working memory task………………………..50
3.4 Visual demonstration of n-back conditions………………………53
3.5 Participants head with fNIRS cap……………………………..55
3.6 Participant position with wearable sensors and feet position…………..56
3.7 Participant’s position during the experiment……………………..57
3.8 Probe locations on the international 10-20 EEG system………………59
3.9 Brain registration maps ……………………………………63
LIST OF ACRONYMS AND ABBREVIATIONS
| ANOVA | Analysis of Variance | |||
| ap | Anterior-Posterior | |||
| AUC | Area Under the Curve | |||
| BA | Brodmann Area | |||
| CDC | Centers for Disease Control and Prevention | |||
| CoM | Center of Mass | |||
| CoP | Center of Pressure | |||
| CT | Computed Tomography | |||
| DLPFC | Dorsolateral Pre-frontal cortex | |||
| EEG | Electroencephalogram | |||
| FA% | False Alarm rate percentage | |||
| FC | Frontal Cortex | |||
| FEF | Frontal Eye- Fields | |||
| fMRI | functional Magnetic Resonance Imaging | |||
| fNIRS | functional Near-Infrared Spectroscopy | |||
| GC | Granger Causality | |||
| GEE | Generalized Estimating Equations | |||
| H% | Hit rate percentage | |||
| HbO | Oxygenated Hemoglobin | |||
| HbR | Deoxygenated Hemoglobin | |||
| HbT | Total Hemoglobin | |||
| IPL | Inferior Parietal Lobule | |||
| M1 | Primary Motor Area | |||
| MC | Motor Cortex | |||
| MEG | Magnetoencephalography | |||
| ml | medio-lateral | |||
| MoCA | Montreal Cognitive Assessment | |||
| MPFC | Medial Pre-frontal Cortex | |||
| NIRS | Near-Infrared spectroscopy | |||
| PAR-Q | Physical Activity Readiness Questionnaire | |||
| PC | Parietal Cortex | |||
| PET | Positron Emission Tomography | |||
| PFC | Pre-Frontal cortex | |||
| PMC | Pre-Motor Cortex | |||
| PPC | Posterior Parietal Cortex | |||
| ROIs | Regions of Interest | |||
| RT | Response time | |||
| SAC | Somatosensory Association Cortex | |||
| SMA | Pre-Motor and Supplementary Motor Area | |||
| SPECT | Single Photon Emission tomography | |||
| SPL | Superior Parietal Lobule | |||
| SPM | Statistical Parametric Mapping | |||
| TBI | Traumatic Brain Injuries | |||
| TC | Temporal cortex | |||
| tDCS | Transcranial direct current stimulation | |||
| TMS | Transcranial Magnetic Stimulation | |||
| TOMAL-2 | Test Of Memory And Learning-2 | |||
| WAV | Waveform | |||
I. INTRODUCTION
Aging has been shown to be associated with a decline of cognitive and motor abilities in humans. With aging, mobility impairments in gait and postural control increase the risk of falls and can be a serious health challenge for the elderly (Holtzer, Epstein, Mahoney, Izzetoglu, & Blumen, 2014; Horak, Shupert, & Mirka, 1989; Overstall, Exton-Smith, Imms, & Johnson, 1977). The Centers for Disease Control and Prevention (CDC, 2017) has reported that 1 in 4 old adults fall every year and most falls go unreported. Indeed, about 30% of the individuals who experience falls annually have been reported to have suffered moderate to severe injuries. These injuries range from head injuries such as traumatic brain injuries, joint sprains, bone fractures such as hip fractures, and post-fall anxiety (Alexander, Rivara, & Wolf, 1992; CDC, 2016; Chang et al., 2004; Manning, Ayers, Jones, Bruce, & Cohen, 1988; Sattin et al., 1990; Tinetti, Speechley, & Ginter, 1988).
Overall, there is sufficient evidence to indicate that falls contribute to loss of mobility, independence, increased hospitalization costs, financial burden and risk of death (Alexander et al., 1992; Carter, Kannus, & Khan, 2012; Magaziner, Simonsick, Kashner, Hebel, & Kenzora, 1989; Rubenstein, 2006; Stevens, Corso, Finkelstein, & Miller, 2006; Tinetti et al., 1988), suggesting a need for effective fall-prevention interventions. However, before an intervention can be developed, the etiology of falls and factors contributing to falls must be clearly understood. One such risk factor is poor postural control (Lajoie & Gallagher, 2004).
Normal postural control is defined as the control of the body’s position in space for the purposes of stability and orientation (Herold et al., 2017; Horak, 2006; Shumway-Cook & Woollacott, 2017). Postural control measured during quiet stance is known as static postural control and if measured during external perturbations or movement is known as dynamic postural control. Researchers have observed that static postural control measures, such as the magnitude of the center of pressure (CoP) sway and the sway velocity, are significantly associated with future falls (Fernie et al., 1982; Piirtola & Era, 2006; Woollacott, 2000). Where, CoP is the point of application of the resultant vertical forces acting on the individual’s base of support (Santos & Duarte, 2016; Shumway-Cook & Woollacott, 1995; Woollacott & Shumway-Cook, 2002a).
In a natural setting, human postural control is maintained while performing other posture-unrelated cognitive tasks such as talking, walking, listening, reading, and typing. This process of maintaining postural control as a primary task while performing a secondary task is known as dual-tasking (Huxhold, Li, Schmiedek, & Lindenberger, 2006 Moghadam et al., 2011; Roalf et al., 2013; Ruhe, Fejer, & Walker, 2010). Dual-tasking can be influenced by modifying either the primary task or the secondary task (Moghadam et al., 2011; Roalf et al., 2013; Ruhe et al., 2010). Previous research by Li et al., 2010 and Smith-Ray, Makowski-Woidan, & Hughes, 2014, have shown that cognitive training using dual-tasks while performing a non-motor secondary task may improve postural control in some old adults but not all. However, there is lack of research on the extent to which modifying cognitive load, as a secondary task, can influence postural control in old adults.
Understanding the impact of cognitive load on postural control may help clinicians better design postural control interventions, in turn minimizing the risk of falls among old adults. For example, to optimize postural control training, it is important to increase instability in the system and therefore increase the CoP sway area (Mckeon et al., 2008; Smania et al., 2010). A protocol that progressively increases cognitive load may identify a load threshold that increases CoP sway area and optimizes the prescription of postural control training using a dual-task paradigm. Changes in cortical activity during the dual-tasking, provide an additional perspective into the cognitive side of the postural control system (Huxhold et al., 2006; Jensen & Tesche, 2002; Rypma, Berger, & D’Esposito, 2002).
There has been increasing evidence suggesting a role for the frontal cortex (FC) (Fujita, Kasubuchi, Wakata, Hiyamizu, & Morioka, 2016b), specifically the Prefrontal Cortex (PFC), in human bipedal postural control (a detailed review is provided by Jacobs & Horak, 2007; Mihara, Miyai, Hatakenaka, Kubota, & Sakoda, 2008). The PFC is also known to play an important role in processes like working memory capacity, executive functioning, action selection, response inhibition, performance monitoring, cognitive function, information processing, behavioral reorganization and reward-based learning (Carter et al., 2017; Herff et al., 2014a; Kane, 2002; Ridderinkhof, van den Wildenberg, Segalowitz, & Carter, 2004). Specifically, Fujita et al. (2016) showed that participants had an increased activation in the right Dorsolateral Pre-Frontal cortex (DLPFC) when they performed a cognitive task that evaluated executive attention and information processing (Stroop task) during a quiet stance task (bipedal and unipedal stance).
Furthermore, PFC has shown to be active and to play a role in the processing of memory and mental workload tasks (Carter et al., 2017; Herff et al., 2014a). For example, Herff et al. (2014) used a working memory task called the n-back to determine if the working memory load was associated with PFC activation using the neuroimaging technique called the functional Near-Infrared Spectroscopy (fNIRS). An n-back task requires for the recall of a visual or auditorily presented stimuli, whenever a stimulus is the same as the one that appears n steps earlier in the sequence. The cognitive difficulty of the task increases as a function of the load factor n. Here, Herff and colleagues useda visual letter recall of increasing load of 1, 2, and 3-back conditions. They found that the hemodynamic responses measured in the PFC were consistently different for the three different workloads. Specifically, highest activation was observed during the 3-back tasks compared to the rest and 1-back tasks. This helped them conclude that PFC plays a role when the memory load is modified (more details on Herff et al. (2014) study is provided in chapter 2).
In addition to the cortical changes, individual perception of cognitive difficulty can be measured by analyzing the behavioral responses. The n-back behavioral measures typically used are response time (RT) and accuracy (Barth, Strehl, Fallgatter, & Ehlis, 2016; Gordon, Breeden, Bean, & Vaidya, 2014; Herff et al., 2014b; Owen, McMillan, Laird, & Bullmore, 2005a). Specifically, studies have shown a decline in accuracy as the cognitive load of the n-back increases from 1-back to 3-back (Carter et al., 2017; Jaeggi et al., 2003). Additionally, these same studies showed an increase in response times with increasing cognitive load from 1-back to 3-back.
The extent to which cognitive load affects static postural control in old adults is unclear. Hence, the current study is designed to specifically address: (A) changes in behavioral responses measured as accuracy with increasing working memory load (B) the effect of increasing working memory load on static postural control (C) the effect of increasing working memory load on cortical changes (D) the differences between cortical and postural measures in healthy young and old adults. The study will use a dual-task paradigm along with specific methodological and theoretical considerations.
Behavioral responses will be measured as reaction time (RT) and accuracy (d’) using a handheld clicker. Postural control measures will be estimated as CoP sway area and sway velocity which will be recorded by two wearable sensors (APDM, Inc.), which has been found to be valid and reliable (El-Gohary, Peterson, Gera, Horak, & Huisinga, 2017; Sankarpandi, Baldwin, Ray, & Mazzà, 2017a; Washabaugh, Kalyanaraman, Adamczyk, Claflin, & Krishnan, 2017) measure for assessing postural control (Horak & Mancini, 2013; Horak, King, & Mancini, 2015; Mancini & Horak, 2010a; Mancini et al., 2011), gait (Caldas, Mundt, Potthast, Buarque de Lima Neto, & Markert, 2017; Martori, 2013; Rigsby & Bigelow, 1989; Simoes, 2011; Washabaugh et al., 2017), fall risks (Aziz, Russell, Park, & Robinovitch, 2014; Riva, 2013) and other kinematic measures in healthy and clinical populations (Horak & Mancini, 2013; Horak et al., 2015; Sankarpandi, Baldwin, Ray, & Mazzà, 2017b). Cortical processes will be measured using fNIRS which will allow for a non-invasive estimate of concentration changes in cortical blood oxygenation (HbO) for multiple cortical regions (frontal, motor and parietal).
Purpose
The purpose of this study is to quantify the effect of cognitive load (as measured by a complex working memory task) on postural control and cortical activity in healthy young and old adults. Specifically, the following research questions will be addressed:
- To what extent does the increase of cognitive load influence postural control in young and old adults?
- What is the hemodynamic response in the frontal, motor and parietal cortical regions with increasing cognitive load in young and old adults and how do activations in these regions relate to each other during our tasks?
- What is the relationship between the cortical activation levels in frontal, motor and parietal regions and the static postural control measures in young and old adults?
In accordance with these research questions, the following potential outcomes are proposed:
- To what extent does the increase of cognitive load influence postural control in young and old adults?
To this date, the direct influence of cognitive load, as measured through an n-back cognitive task, on postural control has not been explored. The CoP sway area given as 95% ellipse area is commonly used to measure postural control. Various studies have included CoP sway area and sway velocity as primary measures to assess standing postural control or sway on land (Moghadam et al., 2011; Ruhe et al., 2010). Additionally, Pellecchia (2003) showed that CoP sway area increased when attentional demands were higher in a cognitive task (Pellecchia, 2003a).
Based on this evidence the hypothesis is that increasing cognitive load would affect the CoP sway area and sway velocity. Specifically, with a higher cognitively demanding n-back, a greater CoP sway area and sway velocity is expected.
- What is the hemodynamic response in the frontal, motor and parietal cortical regions with increasing cognitive load in young and old adults and how do activations in these regions relate to each other during our tasks?
Given the body of evidence for fNIRS using working memory tasks (Fujimoto et al., 2014; Fujita et al., 2016b; Mihara et al., 2008), with increasing cognitive load, I expect to see increased HbO in the frontal and motor ROI, specifically the Dorsolateral Pre-frontal cortex (DLPFC), Medial Pre-frontal Cortex (MPFC), Pre-Motor and Supplementary Motor Area (SMA) and Primary Motor Cortex (M1).
For the parietal region, specifically the Somatosensory Association Cortex (SAC) located posterior to M1, our findings would be the first to explore and report activation changes influenced by changing working memory demands and postural control. The evidence clearly pinpoints the role of parietal regions for sensory control, planning, decision-making and execution of motor actions (Culham & Valyear, 2006). Evidence to support the above hypothesis is additionally strengthened by the work by Rosso and colleagues (2017), who recently observed that attention-demanding cognitive tasks when performed alongside dynamic postural control tasks (single and dual-tasks) resulted in greater activation. This was reported as increased HbO concentration levels in old adults but not in young adults (Rosso et al., 2017). Therefore, an increased HbO concentration value is expected in the SAC with increasing cognitive load.
- What is the relationship between the activation levels of frontal, motor and parietal cortical regions and the static postural control measures in young and old adults?
Given the existing literature on the neural correlates of working memory and postural control, I expect to see an overlap in the cortical regions involved in these processes. I hypothesize that, the HbO levels for the frontal, motor and parietal regions will exhibit a significant relationship with the CoP sway area and sway velocity with increasing working memory load as evidenced from the previous work (Carter et al., 2017; Gordon et al., 2014; Herff et al., 2014a; Owen, McMillan, Laird, & Bullmore, 2005b).
Assumptions
The assumptions of this study are as follows:
- I assume that the CoP sway area and sway velocity will be best measures as predictors of falls. These measures have been chosen as the primary dependent measures as it has been widely used to assess postural control during double-leg stance on land (Ruhe et al., 2010), and also when cognitive load is increased in elderly (Moghadam et al., 2011).
- I assume that the oxygenated concentration values (HbO) will be the best measure to reflect the changes in cortical regions during a changing cognitive load task. HbO has been widely reported in various fNIRS studies (Herff et al., 2014a; Holtzer et al., 2011; Koenraadt, Roelofsen, Duysens, & Keijsers, 2014a; Leff et al., 2011) to detect cortical changes and a valid measure to test working memory and postural control. Therefore, HbO will be considered as the primary dependent measure to show changes in cortical changes during a functional task.
Definitions of Key Terms
- Postural control: The control of the body’s position in space for the purposes of balance and orientation (Herold, Orlowski, et al., 2017; Horak, 2006).
- Static Postural control: Postural control measured during quiet stance
- Dynamic Postural control: Postural control measured during a postural perturbation or movement.
- Center of Pressure (CoP): The point of application of the resultant vertical forces (Fx, Fy, and Fz) acting on the individual’s base of support (Santos & Duarte, 2016; Shumway-Cook & Woollacott, 1995; Woollacott & Shumway-Cook, 2002a).
- Working memory: A cognitive system for the temporary storage and manipulation of remembered information (Baddeley & Hitch, 1974; Baddeley, Logie, Bressi, Sala, & Spinnler, 1986; Mansouri, Rosa, & Atapour, 2015).
- Dual-tasking: The process of regulating postural stability while performing a specific concurrent posture-unrelated cognitive task (Huxhold et al., 2006).
- Attention: The behavioral and cognitive process of selecting discrete subjective or objective information while ignoring other perceivable information (Sarter, Givens, & Bruno, 2001).
II. LITERATURE REVIEW
The purpose of this chapter is to provide a theoretical and applied overview of human postural control, working memory, the influence of working memory on postural control and the neural correlates of postural control and working memory. Specifically, in this chapter, I first introduce and discuss postural control, postural control measures and the technology used to acquire these measures. Secondly, I discuss working memory and the n-back task with changing cognitive load. Further, I discuss the relationship between postural control and working memory systems and the tools used to measure the interaction between these systems. Additionally, the tools used to obtain fNIRS and postural control measures namely the fNIRS imaging system (Hitachi ETG-4000 two-probe Optical Topography System) and the Opal wearable sensors by APDM, Inc., respectively are discussed. Finally, the neural correlates of postural control and working memory and their interaction are discussed.
Postural control
Human postural control is a complex skill which involves the interaction of dynamic sensorimotor processes (Herold, Orlowski, et al., 2017; Horak, 2006). Postural control is defined as “the control of the body’s position in space for the purposes of balance and orientation” (Shumway-Cook & Brauer, 2000; Woollacott, Shumway-Cook, & Nashner, 1986). Maintenance of postural control involves the processing and integration of different sensory information. Over the years, researchers have become aware that human postural control is simply not the ability to react or a compilation of many reflexes but instead is a continuum of the central nervous system exploring the limits of stability. Normal postural stability is defined as the ability to maintain the body’s Center of Mass (CoM) within the base of support with minimal postural sway (Shumway-Cook & Woollacott, 1995; Woollacott et al., 1986).
Various studies have identified that postural control is affected by age and has shown to degrade with age (Campbell, Borrie, & Spears, 1989; Dunn, Rudberg, Furner, & Cassel, 1992; Stelmach, Teasdale, Di Fabio, & Phillips, 1989; Tinetti et al., 1988; Woollacott et al., 1986). Additionally, age-related disabilities and specific type of illness negatively impact postural control. This further increases the risk of falls and in turn the poor quality of life (Horak, Shupert, & Mirka, 1989; Lipsitz, Jonsson, Kelley, & Koestner, 1991; Stelmach & Worringham, 1985). Therefore, there is a need to understand and explore ways to improve postural control in humans (Santos & Duarte, 2016).
Human postural control has been evaluated by using several different paradigms to understand the influence of cognitive, motor and sensory components. Specifically the review by Oliveira et al (2008) explains the need to consider various factors to evaluate postural control in hemiparetic stroke patients. Here, they emphasize the role of various physiological systems involved in postural control which include the sensory afferents, verticality perceptions, movement strategies, cognitive processing and biomechanical constraints to understand the abnormalities associated with stroke and to improve stroke diagnosis (Barros de Oliveira, Torres de Medeiros, Frota, Greters, & Conforto, 2008). Postural control can be evaluated using a variety of quantitative measures. One important and most commonly used is the Center of Pressure (CoP). CoP is defined as “the point of application of the resultant vertical forces (Fx, Fy and Fz) acting on the individual’s base of support” (Santos & Duarte, 2016; Woollacott & Shumway-Cook, 2002b). For decades, CoP has been measured using force platform which quantifies the CoP displacement with respect to the individual’s orientation and outputs a time-series of CoP displacement data in the anterior-posterior (ap) and medio-lateral (ml) directions in centimeters (cm). This process of measuring the CoP is termed as stabilography or posturography. Although inferences related to CoP measures is highly debated and has mixed conclusions, the CoP sway area given as 95% ellipse area and the sway velocity are reliable measures to interpret human postural control. The CoP sway area has been used widely in previous studies to assess standing postural control or sway on land in healthy old and clinical populations (Gray, Ivanova, & Garland, 2014; Moghadam et al., 2011; Qiu & Xiong, 2015; Ruhe et al., 2010) with high reliability coefficients of 0.86 (Moghadam et al., 2011).
Wearable sensors
In the recent years, technological advancement has allowed measuring postural control measures using wearable sensors (APDM Inc, http://apdm.com) placed at different locations in the body. The Mobility Lab Software analyzes the data immediately after the completion of the trial, making the measuring system efficient, less time consuming and quick post-data analyses.It comes with four plugins: Instrumented Timed-up and go (ITUG), Instrumented Sway (ISway), Instrumented Stand and Walk (ISAW), and Instrumented Long Walk (IWalk) (Horak et al., 2015; Mancini & Horak, 2010a).
Three striking advantages of using wearable sensors versus traditional measurement tools such as force plate and motion capture systems (VICON) (Horak et al., 2015) are as follows:
- Impairment-level metric characterization: wearable sensors allow for a better understanding of how and why postural control and gait-related activities are impaired during real-world tasks.
- Wearable sensors have increased sensitivity to postural control and gait measurements which help us understand subtle changes and instabilities during the day to day tasks.
- Wearable sensors provide immediate biofeedback to patients to help them understand the types of changes they need to make and provide immediate learning tools.
- Individual differences in level of impairment is an important issue that wearable sensors accounts for. This allows for clinicians to customize assessments for each patient.
The body of evidence is growing where researchers are using wearable sensors to assess postural control and gait in healthy and clinical populations. For example, Mancini et al., 2014 discuss the need for better assessments for postural control and gait specifically, for individuals with stroke, Parkinson’s diseases, multiple sclerosis, risk of falls and orthopedic disorders. Given the above advantages of wearable sensors, it has proven to be a promising assessment tool for postural control, gait and other mobility disorders. The mobility lab analyses measured using wearable sensors have high sensitivity and specificity scores. For example in a pilot study by King & Horak, 2009, where 20 Parkinsons participants were tested using various assessments (Berg Balance Scale and UPDRS III) and mobility lab, they found that the wearable sensor measures were specific in the medio-lateral sway and turning tasks. These measures did not change in the other assessment and were picked up by the wearable sensors. Additionally, Sankarpandi et al., 2017b tested the reliability of the wearable sensors test measures in 27 patients with vestibular disorders. Sankarpandi and colleagues reported a good within and between sessions reliability with a mean Intra-Class Correlation (ICC) values of 0.76 and 0.71 for the ISway plugin.
Dual-tasking and attention
It is clear that paying attention to two or more tasks is always costly, where one of the tasks is mostly compromised. Where, attention can be defined as the behavioral and cognitive process of selecting discrete subjective or objective information while ignoring other perceivable information (Sarter et al., 2001). The effect of attention on postural control has been mostly evaluated using a dual-task paradigm (Boisgontier et al., 2013; H. J. Huang & Mercer, 2001; Rao, Uddin, Gillman, & Louis, 2013). A dual-task paradigm requires individuals to simultaneously perform two independent tasks that have separate goals. Here, each task can be measured independently as single tasks. Camicioli et al (1997) found that simultaneous performance of verbal fluency tasks during walking slowed the walking speed in patients with Alzheimer’s disease compared to healthy elderly (Camicioli, Howieson, Lehman, & Kaye, 1997). Dual-task paradigms are commonly used to assess cognitive and executive functioning. Additionally, it is also used to assess gait and fall risks.
Different models and theories have been proposed to explore the effects of attention on performance such as postural control and gait (Horak et al., 1989; Lacour, Bernard-Demanze, & Dumitrescu, 2008; Woollacott, 2000; Woollacott & Shumway-Cook, 2002; Yardley, 2001).
Theories of attention
The limited capacity theory by Neumann (also known as general capacity theory or pool resources theory (Pellecchia, 2003b; Riley, Baker, & Schmit, 2003) postulates that the brain has a limited capacity or resources that are allocated for information processing. An example of a study supporting this theory is one where healthy, young participants showed declines in balancing tasks when they were asked to perform a mental task simultaneously. These led researchers to propose that individuals exhibit varying attentional capacity. The limits of this attentional capacity can get affected due to factors such as aging, brain damage, changes in postural control demands (such as making the balancing task hard) or individuals with postural control impairments. However, it is still unclear if the limits to the attentional capacity is influenced primarily due to poor postural control or due to impairments in attentional processing. The motor control interference theory proposes that in a dual-task paradigm, concurrent tasks may compete for processing resources. It is likely that we see an interference during a balancing act while simultaneously performing a cognitive task due to the two systems competing for the common resources (to maintain an upright postural control while performing the cognitive task accurately). Finally, a “selection for action” theory by Allport proposes an action-oriented approach to attention. This theory explains the idea of coupling relevant sensory information to successfully execute an action. Allport explains that in a dual-task paradigm, the execution of a goal-directed action (in our case, maintaining an upright postural control) requires two or more subsystems (in our case cognitive and auditory processing) to modify successfully such that the action is executed successfully. Failing which, one system is given priority over the other.
Working memory
Working memory is defined as “a cognitive system for the temporary storage and manipulation of remembered information” (Baddeley & Hitch, 1974; Baddeley et al., 1986; Mansouri et al., 2015). Working memory is a type of short-term memory that supports ongoing and upcoming behaviors and stores information across different delay periods (Mansouri et al., 2015).
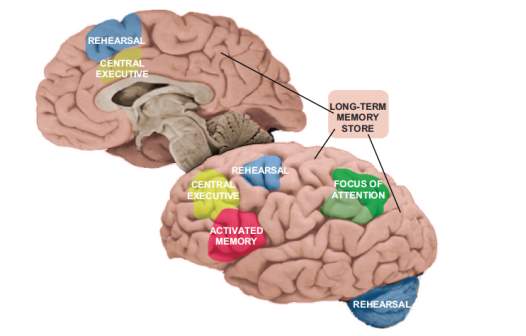
Figure 2.1 Represents the revised neuroanatomical model of working memory given as Embedded-processes model based on a controlled attention theory of working memory (Chein & Fiez, 2010).
n-back working memory paradigm
n-back has been used as a neuropsychological assessment tool to test working memory. A quantitative meta-analysis of 24 fNIRS studies by (Owen et al., 2005b) reviewed the neural correlates of n-back working memory paradigm that used either verbal or nonverbal stimuli. This review concluded that consistent significant activations were observed in frontal, motor and parietal regions while performing n-back tasks. Braver et al., 1997 conducted the first preliminary study using fMRI to investigate the neural correlates with increasing cognitive load (n-back = 0,1,2,3). They found that young healthy adults (8 males and 1female) showed a linear increase in the dorsolateral and the inferior Prefrontal Cortex (PFC) activation. Additionally, they found that brain regions other than the PFC are also involved in the processing of increasing cognitive load.
An n-back task involves identifying or locating a series of verbal (auditory) or non-verbal (visual) stimuli where an individual needs to indicate when a specific stimulus is the same as the one presented n trials before that stimulus (Owen et al., 2005b). n-back task is one of the most popularly used tasks to test the working memory paradigm in various behavioral and neuroimaging studies such as fNIRS (Dommer, Jäger, Scholkmann, Wolf, & Holper, 2012; Herff et al., 2014a), fMRI, EEG. A detailed review of different types of n-back used in 24 neuroimaging studies has been review by Owen et al., 2005.This meta-analysis reported that a robust activation is observed in six cortical regions: bilateral Premotor cortex (PMC), Supplementary Motor Area (SMA) the PFC and the bilateral and medial Posterior Parietal Cortex (PPC). Within these main regions they saw activation specifically in the lateral, dorsal cingulate and medial premotor cortex; the dorsolateral and ventrolateral PFC and the medial and lateral PPC (Owen et al., 2005b).
The PFC has shown to be active and to play a role in the processing of memory and mental workload tasks (Carter et al., 2017; Herff et al., 2014a). For example, Herff et al. (2014) used a working memory task called the n-back to determine if the working memory load was associated with PFC activation. The authors used a visual letter recall of increasing load of 1, 2, and 3-back conditions. Here they were asked to respond when the current letter matches the letter from n-steps earlier. Herff and colleagues used an event design where each letter was visually presented for 2000 ms. The letter presentation time was for 500 ms and participants had 1500 ms to respond accurately. Each trial consisted of 22 letters and they used 30 trials of 64 s and 15 rest trials of 10 s. Their results showed that the hemodynamic responses measured in the PFC were consistently different for the three different workloads. Specifically, highest activation was observed during the 3-back tasks compared to the rest and 1-back tasks. This helped them conclude the role of PFC in changing working memory load. However, some of the pitfalls of this study were that they did not control for the left and right-handed participants. Additionally, the study had a small target sample of 22 letters. Further, they only reported the grand averages for the hemodynamic response for oxygenated and deoxygenated blood concentration values for all 8 channels. By doing so, they may have oversimplified the cortical activation data. Lastly, they used an event design but a block design would have strengthened this study. This is because a block design (a) uses a trial-by-trial framework, (b) allows for efficient ways to compare multiple trials between blocks thereby reducing signal-to-noise ratio, (c) is designed to detect changes based on Regions of Interest (ROIs) (Dale & Buckner, 1997; Maccotta, Zacks, & Buckner, 2001).
Neuroanatomy
Cortical Regions of Interest (ROI)
Before one dives into the specific neural correlates of human postural control and working memory, it is important to get familiarized with the neuroanatomy of movement and cognition. Specifically, the neuroanatomy of postural control and working memory. In this section, the basics of the human cortex, primary cortical regions and their role in the postural control and working memory is explained.
The specific cortical regions of interest (ROI) pertaining to this study are the Frontal Cortex (FC, Figure 2.2), Motor Cortex (MC, Figure 2.5) and the Parietal cortex (PC, Figures 2.6 & 2.7) for the right and left hemispheres in the human brain. These regions are primarily associated with postural control and working memory and have been extensively studied in the literature. For the purpose of this study, specific regions of the cortex known as the ROIs are chosen.
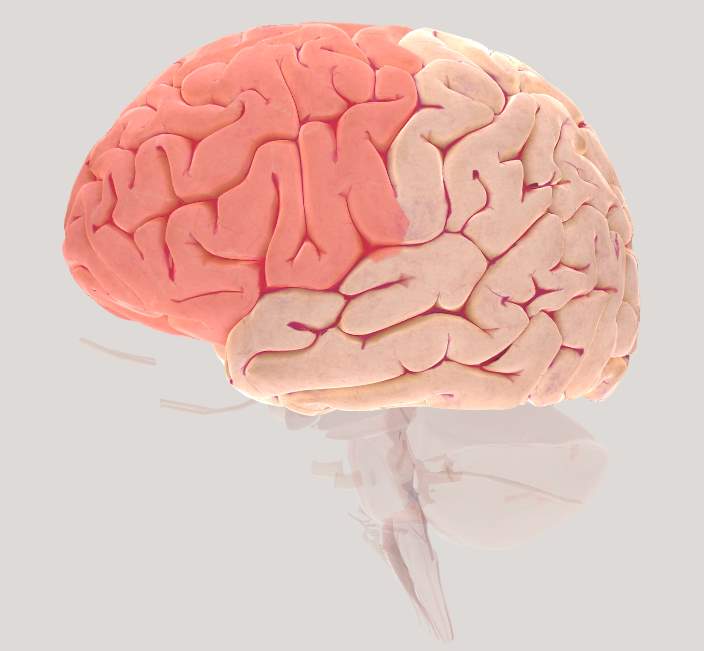
Figure 2.2 Brain view illustrating left Frontal cortex (FC)
Prefrontal Cortex (PFC)
The prefrontal cortex (Figure 2.2) is a part of the frontal cortex (FC) and has shown to support high-level control processes collectively known as “executive functions”. Additionally, the prefrontal cortex (PFC) has shown to be involved during postural control (Bolton, 2015; Ferrari et al., 2014; McKendrick, Parasuraman, & Ayaz, 2015; Ridderinkhof et al., 2004). PFC plays an important role not only in basic memory processes but also in planning and execution of sequential behavior that span longer than single action plans which is generally termed as “working memory” (Boschin & Buckley, 2015). This has been specifically shown at cellular level by several studies where cell structures encode stimulus features, regulate maintain and manipulate information over time (Goldman-Rakic, 2011). Clinical and behavioral studies have shown that damage to the PFC results in deficits with attention, motor control, short-term memory, temporal memory, spatial orientation, and associative learning (for reviews, see (Kane, 2002; Roberts, Robbins, & Weiskrantz, 1998).
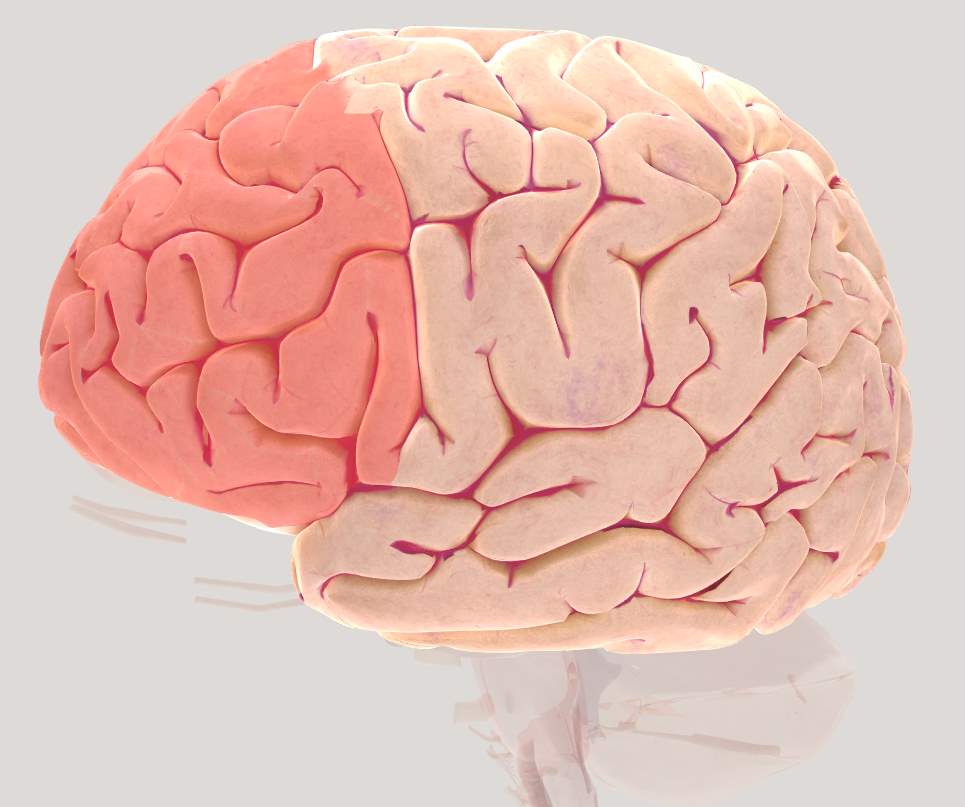
Figure 2.3 Brain view illustrating left Pre-Frontal Cortex (PFC)
Dorso-Lateral Pre-Frontal cortex (DLPFC)
The Dorso-lateral Prefrontal Cortex (DLPFC) also known as Anterior Prefrontal Cortex and is roughly equivalent to BA 9 and 46. Extensive evidence suggests that DLPFC plays an important role in maintaining information across delays. Lesions in DLPFC has shown to be responsible for deficits in tasks involving responding to delays and maintaining online control (Boschin & Buckley, 2015). Additionally, DLPFC has shown to play an important role in working memory function (Fregni et al., 2005).
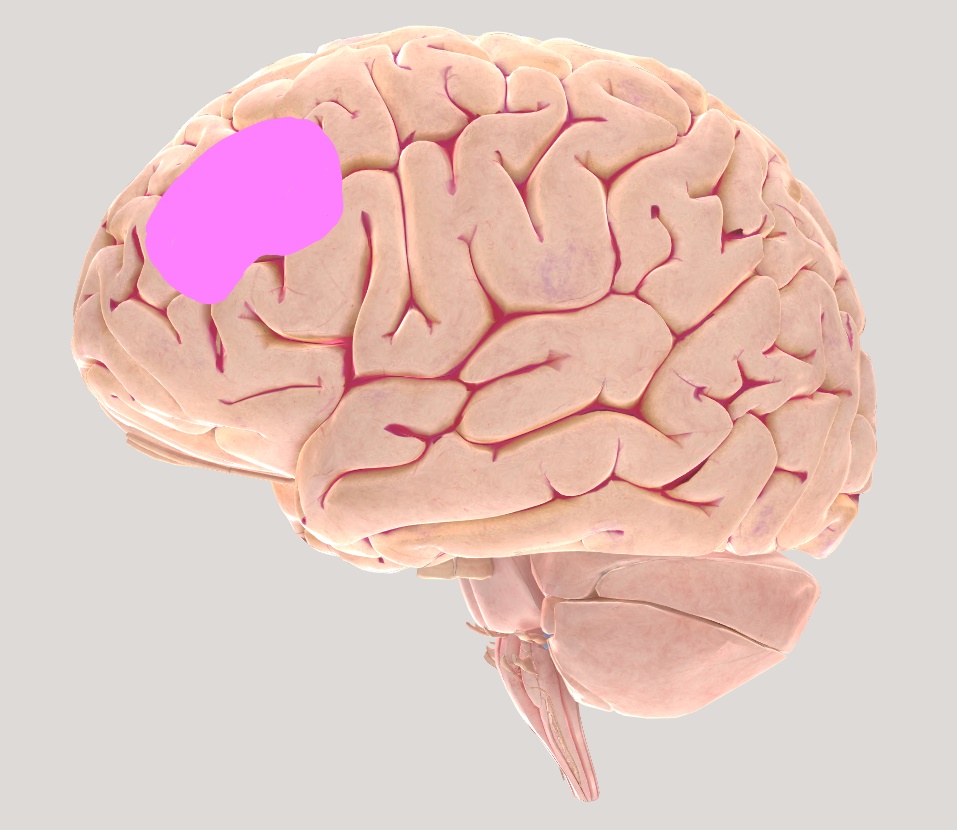
Figure 2.4Brain views illustrating left Dorsolateral Pre-frontal cortex (DLPFC)
Motor Cortex (MC)
The Motor Cortex (MC) consists of the Supplementary Motor Area (SMA) and the Pre-Motor Cortex (M1). The MC receives information from the sensory system and continually communicates with other deeper brain structures such as the basal ganglia, cerebellum and the cortical structures to generate precise, intentional, coordinated movements.
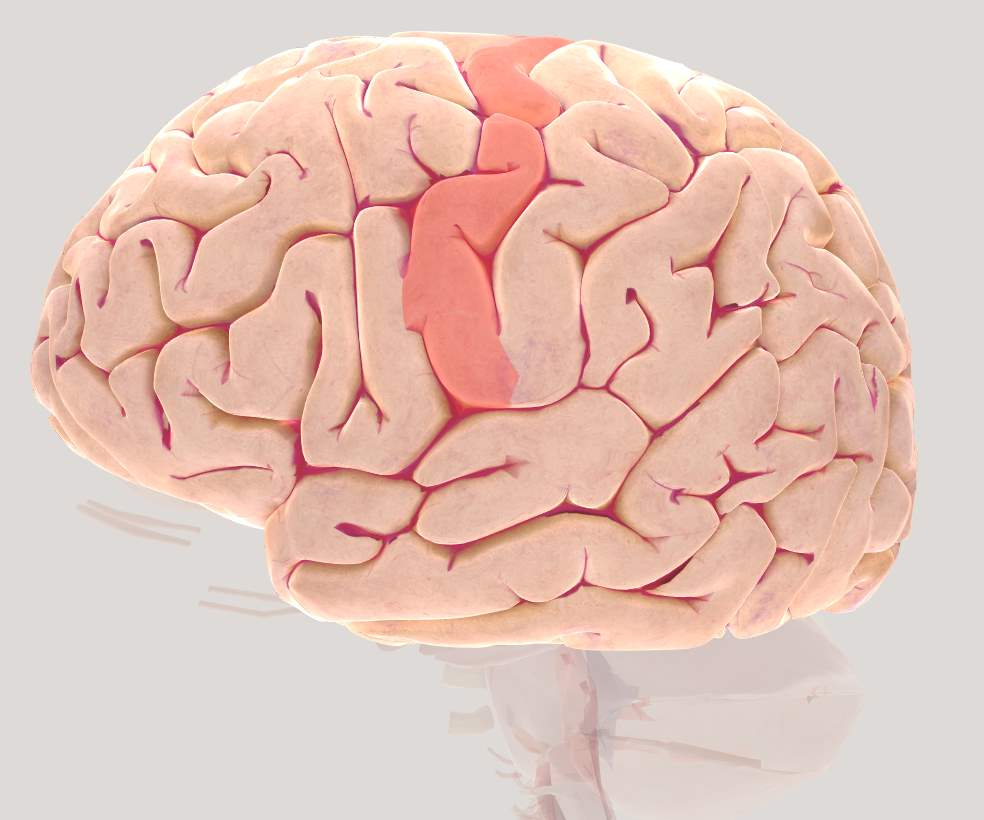
Figure 2.5 Brain view illustrating left Motor Cortex (MC)
Somatosensory Association cortex (SAC)
The SAC is the anterior-most region of the parietal cortex (PC) and maps the touch receptors from various parts of the human body. In this study, this region will play an important role in retrieving postural control information from the receptors in the foot.
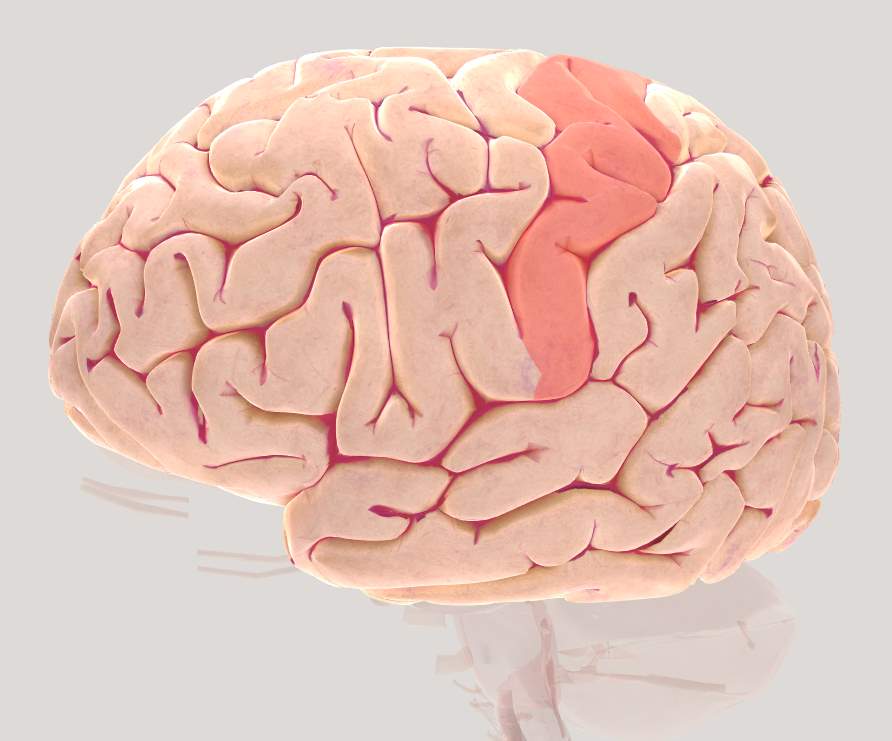
Figure 2.6 Brain view illustrating left Somatosensory Association cortex (SAC)
Neuroimaging tools for functional imaging
Currently, there are seven well-established non-invasive brain-imaging techniques that have been used for various clinical, educational, medical, diagnostic, rehabilitation and research purposes. Functional imaging allows measuring neuronal activity during any functional task. The functional imaging tools currently being used are functional Magnetic Resonance Imaging (fMRI), Computed Tomography (CT), Positron Emission Tomography (PET), Electroencephalography (EEG), Magnetoencephalography (MEG), Transcranial magnetic stimulation (TMS) and Functional Near-Infrared Spectroscopy (fNIRS) (Demitri, 2013). fMRI works on the principle similar to fNIRS where it detects changes in blood oxygenation in response to any neural activity. The greater the activity, which is indicated as red bright areas, determines greater neuronal activity. fMRI currently is the most precise neuroimaging technique in terms of spatial resolution. Which means that not only can it detect neuronal activity in the cortex, it can precisely detect neuronal activity in the subcortical and deep brain regions. However, fMRI has the biggest drawback in terms of its ability to measure cortical activation during tasks involving movement. Additionally, it has a very low temporal resolution. Computed tomography also known as CT scan creates pictures of the brain based on differential absorption of X-rays. This technique allows for obtaining a gross anatomical picture of the brain but does not provide clear structural information. PET imaging technique provides a functional mapping of the brain based on detection of short-lived, trace radioactive material inside the brain. Greater radioactivity implies greater active brain regions. MEG measures brain activity by detecting the magnetic fields produced by the electrical activity of the neurons. MEG is mainly used in research and clinical settings to understand the functions of specific brain structures. It is also used clinically to localize any brain pathology. EEG is one of the most abundantly used imaging technique due to its low cost and non-invasive nature. Additionally, ongoing advancement in EEG imaging systems makes it a popular imaging technique for researchers. EEG allows for collecting data involving movement and functional tasks, however, it has a very low spatial resolution. EEG is most commonly used for tasks that involve high temporal precision due to its high temporal resolution. fNIRS works on the similar principle as fMRI where an infrared light between 700-900 nm detects changes in blood oxygenation and deoxygenation in a given brain Region of Interest (ROI). fNIRS is widely used due to it relation to fMRI but also due to its lower cost and ability to measure brain activity during functional tasks which is impossible in a fMRI (Crosson et al., 2010; Demitri, 2013). This study will use a NIRS system: Hitachi ETG-4000 two-probe Optical Topography System, with a 3×5 channel montage.
Functional Near-Infrared Spectroscopy (fNIRS)
fNIRS is a neuroimaging technique that measures concentration changes obtained as cortical blood oxygenation and deoxygenation levels, through infrared light interaction with the brain tissue of a specific region (Chance et al., 1998; Delpy & Cope, 1997). fNIRS is a non-invasive neuroimaging technique that detects changes in optical properties of the human cortex by providing the regional activity as a map or image. fNIRS is less prone to movement artifacts compared to fMRI and EEG which makes it an excellent tool to measure cortical changes during motor tasks involving fine movements such as speech (Dieler, Tupak, & Fallgatter, 2012), writing , finger movement (Brigadoi, Cutini, Scarpa, Scatturin, & Dell’Acqua, 2012; Koenraadt, Duysens, Meddeler, & Keijsers, 2013) and gross movements such as gait , static and dynamic postural control, driving a car etc. (Holtzer, Epstein, Mahoney, Izzetoglu, & Blumen, 2014a; Holtzer, Wang, & Verghese, 2012; Koenraadt, Roelofsen, Duysens, & Keijsers, 2014b; Maidan et al., 2015; Mirelman et al., 2017) which would be impossible to measure any movement related tasks using fMRI. Also, motoric tasks may result in noisy data if measured using EEG. Hence, fNIRS has become a popular imaging tool to measure long periods of cortical activity during functional movement tasks.
fNIRS uses an infrared light which is emitted by an emitting optode (emitter) on to the human scalp. The concentration changes in the oxygenated hemoglobin (HbO) and deoxygenated hemoglobin is then detected as the light passes through the scalp and is received back by another optode (detector) (Figure 2.8 A & B). This change in the concentration of the light signal gives the amount of oxygenated hemoglobin (HbO), deoxygenated hemoglobin (HbR) and total hemoglobin (HbT = HbO + HbR).

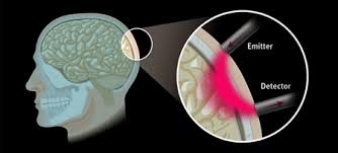
Figure 2.8 (A) fNIRS principle, (B) 3×5 fNIRS probe holder with 8 emitter and 7 detector fiber optic probes.
NIRS-SPM
Statistical parametric mapping (SPM) tool is a statistical program that determines the differences in recorded brain activity by using spatially extended statistical processes for functional neuroimaging experiments. SPM uses MATLAB based functions to model the activations during functional tasks (Man et al., 2015; Ye, Tak, Jang, Jung, & Jang, 2009a).
Neural correlates
Neural correlates of working memory
The role of cortical networks in the control of bipedal stance has been a topic of interest in recent years as a gateway to understanding fall risk in the aging population. Multiple mechanisms and physiological systems underlie postural control. Human cognition involves several cognitive processes such as attention, working memory, decision making and executive functioning that are required for postural control (Litvan, Mohr, Williams, Gomez, & Chase, 1991; Teasdale & Simoneau, 2001). Damage to any of the underlying systems may result in various context-specific instabilities (Horak, 2006). Therefore, it is important to understand the various systems underlying and regulating postural control to create effective assessment and rehabilitation of postural control disorders. Additionally, understanding how an individual’s postural control gets affected gives us further insight into the understanding of assessing and treating postural control disorders.
fNIRS and working memory
fNIRS has proven to be an effective tool in measuring cortical activity during working memory tasks. Specifically, in a recent study using 13 healthy adults that focused on voluntarily upregulating hemodynamic response in the prefrontal regions during a 2 weeks neurofeedback session. Although the study did not show systematic learning in the individuals, they were able to show focused prefrontal activation during pre-training sessions (Barth et al., 2016). Additionally, a Transcranial direct current stimulation (tDCS) study showed that anodal stimulation of the DLPFC resulted in an enhanced performance in working memory tasks but cathodal stimulation did not yield in increased performance.
Neural correlates of postural control
Understanding cognitive processes, attention and executive functions are essential in predicting variability in mobility performance (Holtzer et al., 2012). The relationship between the structure and function of the brain during mobility tasks has gained attention in the recent years and is still in the initial stages of exploration. However, technological constraints in neuroimaging, restrict the process of measuring neural activation during functional tasks such as walking, cycling, running etc. Traditionally, it was believed that only subcortical regions are involved in mobility and postural control. Lesion case study by Nielson has shown that the patient with a selective lesion in the pyramidal tracts bilaterally, was unable to walk up to 5 months after injury but regained the ability to walk later. This evidence demonstrates the role of pyramidal tract in the control of walking (Nielsen, 2003). Early evidence using Single photon emission tomography (SPECT) has shown increased blood flow in the sensorimotor cortex, cerebellum and frontal region during walking in healthy individuals. However, it is unclear if the neuronal activity is solely related to the muscle activity during walking (Fukuyama, Ouchi, Matsuzaki, & Nagahama, 1997). In the recent years with advancing neuroimaging techniques, regions of the cerebral cortex specifically, the PFC have been identified to be involved during postural control (Bolton, 2015; Ferrari et al., 2014; McKendrick et al., 2015; Ridderinkhof et al., 2004). fMRI (Bandettini, 2007), fNIRS (Ferrari & Quaresima, 2012) and EEG (Kober, Kurzmann, & Neuper, 2012) are the widely used neuroimaging modalities to understand the role of cortical regions during various tasks. Although fMRI is the best tool with respect to spatial resolution and EEG for measuring event-related potentials, fNIRS has greater temporal resolution compared to the two, which makes it a perfect neuroimaging tool to assess postural control and mobility tasks. Additionally, there are no fMRI /PET studies that have measured PFC activation during postural tasks as fMRI does not permit measuring brain activity during movement based tasks.
fNIRS and postural control
Excitation of the cortical networks during gait and postural control has been an important topic of research in the recent years. A recent study by Mirelman et al., 2017, testing young and old adults during challenging walking conditions found that old individuals recruited primarily PFC during simple tasks whereas found that both young and old adults showed increased neural activation as the complexity of the task increased. Additionally, they found that old adults showed greater activation of the PFC, which was associated with increased gait variability as well.
Mihara et al. 2008, first provided evidence for activation in the cortical regions during a postural control task using fNIRS. Mihara and colleagues used a 50 channel fNIRS system, where they perturbed fifteen healthy adults with and without auditory warning signals while balancing on a force platform. They observed significant activation in the DLPFC and Frontal eye-fields (FEF) irrespective of the warning signals provided. They concluded from this study that the DLPFC and FEF play an important role in human postural control. Additionally, they argued that the activation in the PFC could have been due to the saccular stimulation (which is a part of the fluid-filled sacs of the labyrinth of the inner ear) by the force platform. This could have indirectly activated the PFC via the afferent input. Furthermore, Mihara and colleagues perturbed the participants in the presence of vision, which could have been another reason for the greater activation of the FEF and DLPFC due to the gaze adjustments and anticipatory visual correction. Here, Mihara and colleagues challenged the postural system by an external perturbation paradigm. Whereas increasing the cognitive load is another way of challenging the postural and the cortical system (Mihara et al., 2008).
Later in 2012, Mihara and colleagues found that cortical networks played an important role in post-stroke patients during a postural control task, which was measured using fNIRS. Recently, Ferrari et al 2014 pinpointed that the PFC showed bilateral activation during a tilt board postural control task. They measured the oxygenated hemoglobin (HbO) response in the PFC using fNIRS in 22 male adults during a 5-min incremental tilt board postural control task in a semi-immersive virtual reality environment mediated by the depth-sensing camera. They found an increased sway and error with increasing task difficulty. fNIRS evidence showed bilateral activation of PFC suggesting its role in balancing tasks involving tilt (Ferrari et al., 2014).
Working memory and postural control
We now know that the simple ability to stand and balance on two feet is not so simple, but a highly cognitive process. Additionally, this ability depends on the information from various sensory systems such as touch, vision, vestibular control etc. (Huang, Mao, Chen, & Li, 2014; Krishnan, Cho, & Mohamed, 2017; Yardley, 2001). Evidence for cognitive processes influencing the postural control system comes from studies where the reaction times to respond to a cognitive task were greater when participants stood quietly versus them sitting with a support. Additionally, we also know that with increasing postural task difficulty, the cognitive processing time increases (Teasdale & Simoneau, 2001).
In summary, working memory processes and postural control shares the same resources, due to which postural control is affected when there is an increase in cognitive load. However, it is unclear how these systems influence each other. Specifically, we do not know the extent to which working memory load influences postural control. Hence, there is a need to understand how these two systems interact and in turn affect cortical changes and postural outcome measures.
III. METHODS
The current study will use a mixed (between and within groups) quasi-experimental design in which two groups of participants will be asked to perform a double-leg stance, postural control task with their eyes closed while simultaneously responding to a control task (standing with their eyes closed) or a working memory (n-back) task. The entire protocol (see Figure 3.1) will consist of two blocks and three rests. Each block will contain the experimental tasks (one control and four n-back) in a pseudo-randomized order. The three seated rest periods will surround the blocks. During all the cognitive tasks, participants will wear a fNIRS cap and two sensors to measure cortical activation and postural control. More details are provided in the experimental design shown in Figure 3.1.
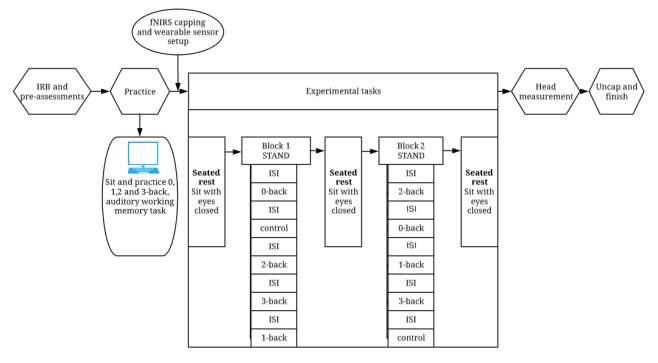
Figure 3.1 Flowchart showing the steps involved in the experiment. The two blocks will have pseudo-randomized orders.
Participants
Two groups: a young group, age range of 18-40 years, and an old group, age range of 60-85 years, of right-handed adults will be recruited. I will collect postural control data (CoP sway and sway velocity) and memory response time (RT) and accuracy (d’) data concurrently as participants complete multiple n-back tasks (0-back, 1-back, 2-back and 3-back). Two independent statistical power analyses were conducted for the postural control (sway) and working memory measures using G*Power (Erdfelder, Faul, Buchner, & Lang, 2009; Faul, Erdfelder, Lang, & Buchner, 2007). For the postural control measures, I plan to conduct a repeated measures ANOVA with two groups and four measurements. Planning for two groups, an alpha of 0.05, a power of 0.80, an effect size (f = 0.20), correlation among the repeated measures of 0.3 and five measurements (control, 0,1,2, and 3-back), the desired total sample size obtained was 48 (Figure 3.2).
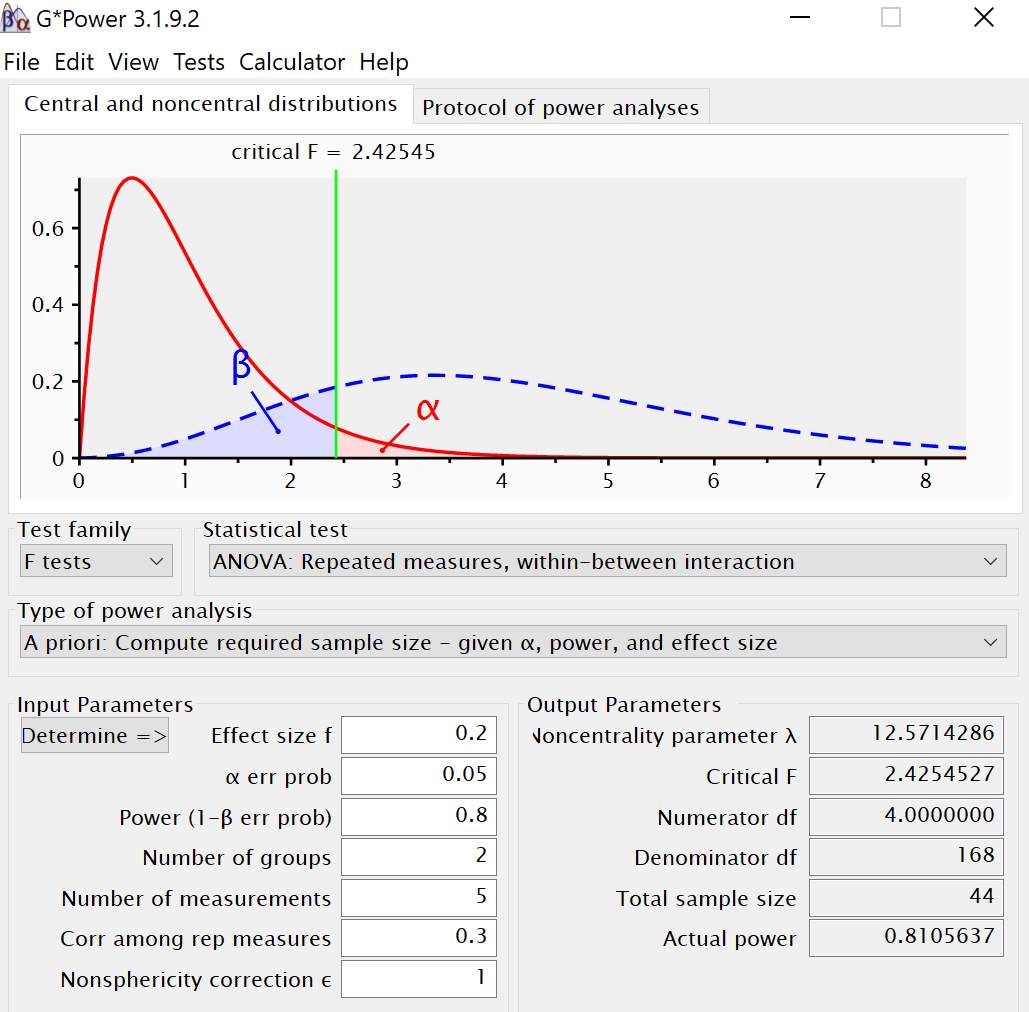
Figure 3.2 Power analysis output from G-Power for postural control CoP sway measure.
For the working memory task, I plan to conduct a repeated measures ANOVA with two groups and four measurements (Control, 0, 1, 2 and 3-back). Using an alpha of 0.05, a power of 0.80, an effect size (f = 0.20), correlation among repeated measures as 0.3 and four repeated measures, the desired total sample size obtained was 50 (Figure 3.3).
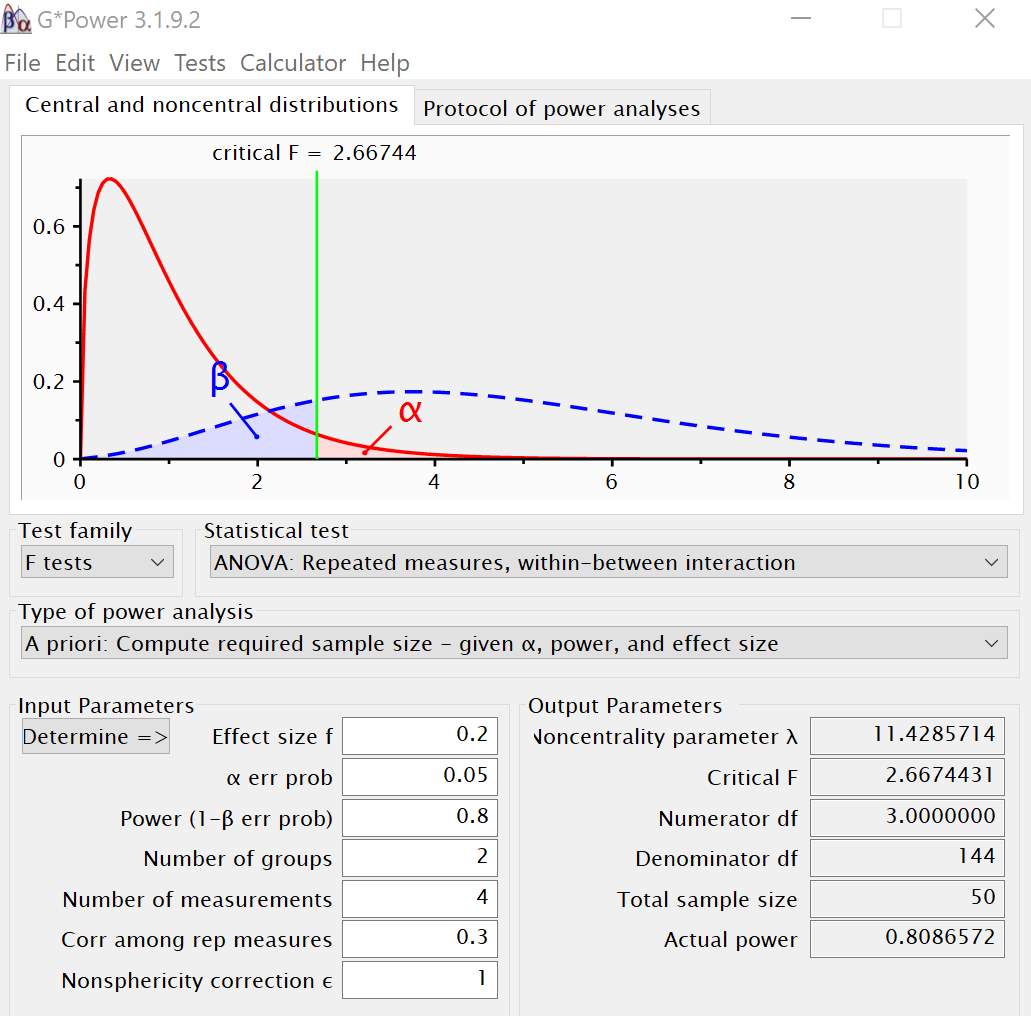
Figure 3.3 Power analysis output from G-Power for working memory task
Previous studies (Ferrari et al., 2014; Fujita, Kasubuchi, Wakata, Hiyamizu, & Morioka, 2016a) that have used postural control and working memory tasks have included 24 participants per group. Given my power estimates (with conservative expectations), I have decided to recruit 24 participants per group into the proposed study. All participants will be recruited from Utah State University campus community and the Cache Valley community.
Procedures
Pre-assessments
The procedure will start with participants signing the informed consent form (IRB #8591) approved by Utah State University Institutional Review Board. Following this, they will complete the Physical Activity Readiness Questionnaire (PAR-Q) (Canadian Society for Exercise Physiology, 2002), the Montreal Cognitive Assessment (MoCA) dementia screening test and the Letters forward short-term memory task (Reynolds and Voress, 2007). The PAR-Q measure, which consists of seven simple questions, assesses the level of physical readiness for individuals in the age range of 15-69 (Shephard, 1988; Thomas, Reading, & Shephard, 1992). Participants will be excluded if they answer “yes” to question 4, “Do you lose your postural control because of dizziness or do you ever lose consciousness?” Participants will also be excluded if they score 13 or below on the MoCA, which would suggest the possibility of Alzheimer’s disease (Roalf et al., 2013). Finally, participants will be excluded if they have any neurological disorders such as Alzheimer’s disease, epilepsy, brain tumors, Parkinson’s Disease, Multiple Sclerosis, and migraines. Additionally, participants will be excluded if they present a lower extremity injury or a history of injury six months prior to the study, or if they are left-handed (to be consistent with previous studies and avoid confound in the neural data). Physical characteristics of all participants will be recorded as shown in Table 3.1.
| Table 3.1 Physical characteristics of all participants (n = 40). | ||||
| Groups | Characteristic | Mean | SD | Range |
| Young (n=20) | Age (yrs) | |||
| Mass (kg) | ||||
| Height (m) | ||||
| MoCA (#) | ||||
| Activity level (days/week) | ||||
| Level of education (1-6)
Letters forward score |
||||
| Old (n=20) | Age (yrs) | |||
| Mass (kg) | ||||
| Height (m) | ||||
| MoCA (#) | ||||
| Activity level (days/week) | ||||
| Level of education (1-6)
Letters forward score |
||||
| MoCA = Montreal Cognitive Assessment; SD = standard deviation | ||||
| Level of education 1 = grade school; 2 = jr high; 3 = high school; 4 = college; 5 = graduate school; 6= degree | ||||
n-back procedure
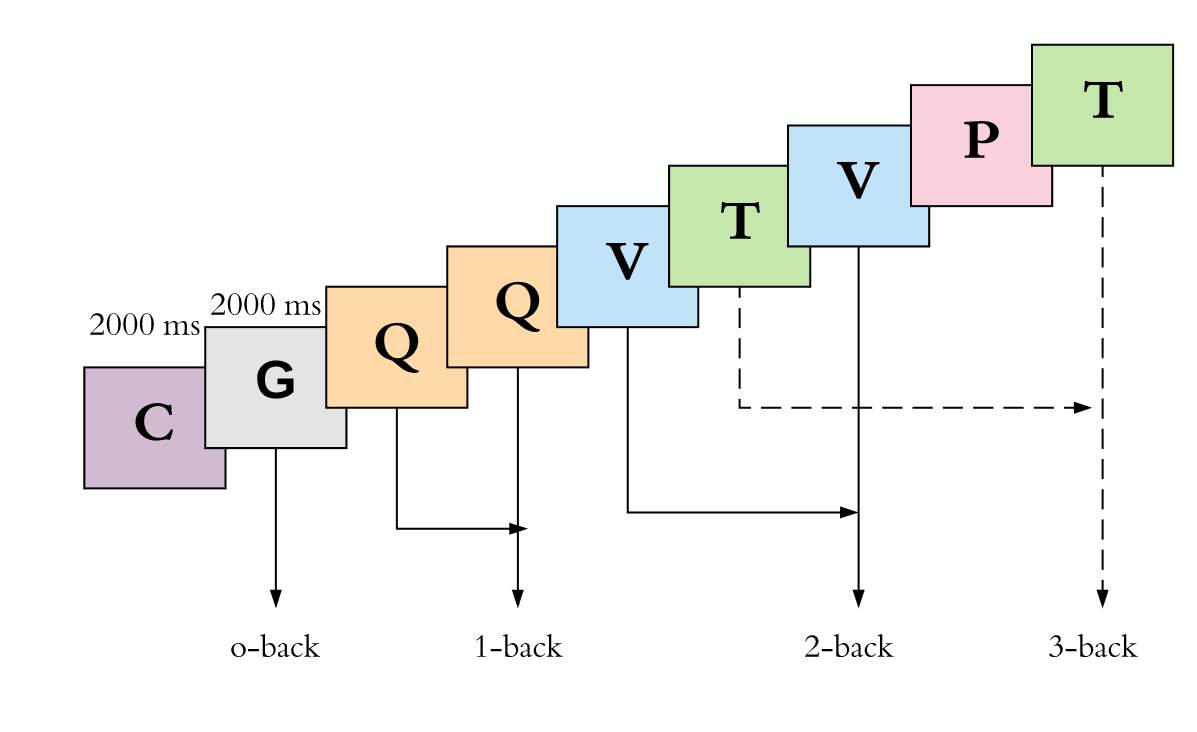
In the n-back task, participants listen to a sequence of auditorily presented letters while wearing an eye-mask to occlude vision. This set-up is designed to ensure no interference from the visual system to influence or stabilize postural control (Mancini & Horak, 2010b; Visser et al., 2008). Participants use a hand-held clicker to indicate whenever a stimulus is the same as the one that appears n steps earlier in the sequence. The cognitive difficulty of the task increases as a function of the load factor n.
There are four conditions: 0-back, 1-back, 2-back and 3-back (Figure 3.4). In the 0-back task, the participant is told to indicate with a finger click whenever s/he hears the letter “G” within a one minute 35-second sequence.
K D Q G B C T V G K D Q G B C
In the 1-back condition, the participant identifies whenever a letter is repeated within a one minute, 35-second sequence.
T D Z Z B Y C C V K T T G Z B
In the 2-back condition, the participant identifies any letter that they heard two items back within a one minute, 35-second sequence.
T V D V C K C Z V K T K G Y B
In the 3-back condition, the participant identifies any letter that they heard three items back within a one minute, 35-second sequence.
Z B G T B K V C P V T P Y D B

Time
Figure 3.4 Visual demonstration of the four n-back conditions
n-back stimuli
All 12 letters (B C D G J K P Q T V Y Z) and instructions for the n-back task were recorded using a Sony IC Recorder (Sony Electronics, Inc., 2007). The waveform (WAV) audio files were exported into an open source digital audio editor (Audacity 2.0.2) and low pass filtered at 6 dB with a cutoff frequency of 1000 Hz. Following the filtering, the WAV file maximum amplitude was normalized to -1.0 dB to remove any DC offset. Each auditory stimulus for the n-back task (spoken letters) was cut to occur within a 2000 ms window.
The trial orders for each n-back task were first randomized with the constraint that each n-back condition included a total of 40 letters and 13 targets. The n-back task was designed using E-Prime 2.0.8.22 (Psychology Software Tools, Inc., Schneider, Eschman, Zuccolotto, & Guide, 2002). All stimuli will be presented auditorily through Logitech speakers at a comfortable volume.
Practice
Participants will complete a practice session in which they become familiarized with the n-back task. Participants will be seated in a chair with an eye mask on while they listen to the n-back instructions presented through Dell speakers. Each practice condition will consist of 15 stimuli with 3 correct responses. In order to ensure that there is no practice effect, the order of the items in the practice conditions are independent of the orders within the experimental conditions. Participants will be instructed to click a button on a clicker (Beboncool RF 2.4GHz, wireless presenter, see Figure 3.4) for each correct response (the target). After the practice session, participants will have the opportunity to ask further questions in case they need any clarification before the experiment begins.
fNIRS capping and wearable sensors setup
A Hitachi ETG-4000 two-probe Optical Topography System, with a 3×5 channel montage, will be used to measure concentration variations of oxygenated and deoxygenated hemoglobin in the anterior left and right hemispheres. Following practice, a fNIRS cap consisting of two 3×5 probes (probe 1 and probe 2, See Figure 3.2) will be placed on the participants’ head. The 2 probe sets will be placed such that the nearest corner of the anterior probe is close to the left and right canthi. This initial calibration is designed to enable us to capture the best coverage of the left and right frontal, motor and parietal regions. A NIR gain check will be performed prior to initiating the fNIRS data recording to ensure that the data is neither under-gained nor over-gained. All fNIRS data will be recorded at 695nm and 830nm.

Probe 2

 Probe 1
Probe 1



Figure 3.5 Participants head superior view with fNIRS cap (probe 1 and probe 2)
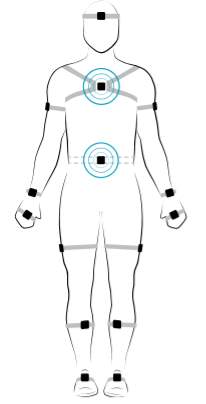
Participants will also wear two wearable sensors (APDM, Inc.) housed in straps. One sensor will be placed on the sternum (chest) and the other on the posterior trunk at the level of Lumbar vertebrae 5 (See Figure 3.3). As part of the practice session, participants will be familiarized with the double-leg stance. They will be asked to stand with their feet together (Figure 3.3), hands on either side of their body and eyes closed.
A



S
L
Wearable sensors

B

Figure 3.6 (A) Demonstration of the Sacral (S) and Lumbar (L) positioning of the wearable sensors on the participant body. The blue circles appear when the wearable sensors are calibrated and ready for data collection. (B) Feet together position used for the current study. This position ensures additional challenge to the static postural control along with no vision and increasing working memory load. This decision was derived from the review discussed by Visser, Carpenter, van der Kooij, & Bloem, 2008, on how various factors when manipulated affect static postural control.
Experimental protocol
The experiment will start with the seated rest, followed by block 1 (while standing), middle seated rest, block 2 (while standing) and an end seated rest (Figure 3.1). The participant position for the two standing blocks of n-back tasks with fNIRS cap and wearable sensors are demonstrated in Figure 3.4.
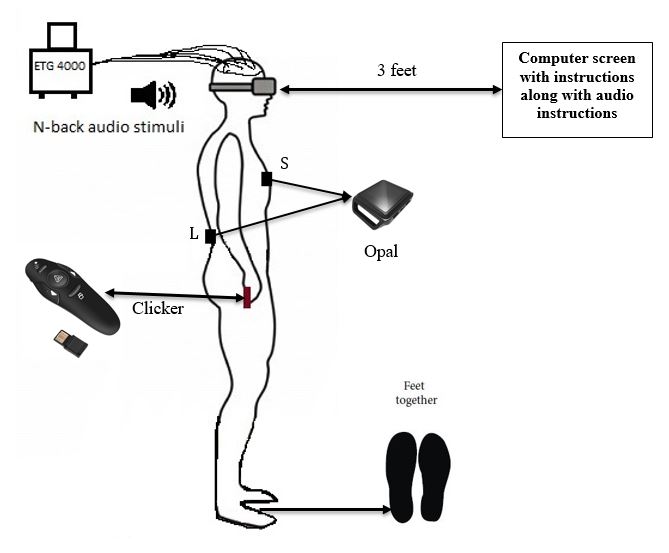
Figure 3.7 Description of participant position with fNIRS and wearable sensors placed on the sternum and posterior trunk at the level of L5 with straps.
During the experiment, each n-back condition will contain an 80-seconds sequence of letters that will be presented in a pseudo-randomized order. Pseudo-randomization is a common methodological consideration for ensuring similar order effects (if present) across groups in cognitive psychological studies (Carter et al., 2017; Goh et al., 2017; Herff et al., 2014a; Miller, Price, Okun, Montijo, & Bowers, 2009). The instructions will be same as practice. However, during the experiment, participants will perform the n-back tasks while maintaining a double leg stance and responding using a clicker.
Head measurement
Once the experiment is completed, participants will be instructed to keep the fNIRS cap on while the examiner carefully removes the optodes. Using a measuring tape, the exact center of the head (Cz) will be first located. Measurements in centimeters will be taken from the left auricular lobule to the right auricular lobule, and from the nasion to the inion. Once the center is determined, a magnet will be positioned on the center of the head, and the participants head will be moved such that the inion is 10cm away from the transmitter. Using the stylus, 5 head base reference points will be measured namely: nasion, left tragus, right tragus, inion, and Cz (See Figure 3.6). After the 5 reference points are measured, all other optical fiber points will be measured in numerical order starting with probe set 1 (ascending order) and ending with probe set 2 (ascending order) (See Figure 3.5).
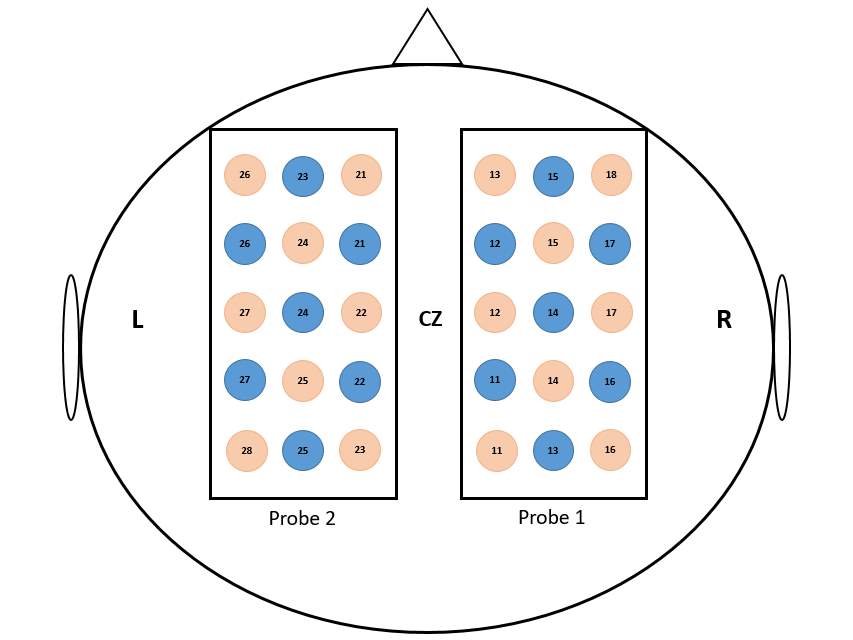
Figure 3.5 Schematic illustration of probe location. The values between the emitter (red circles) and the detectors (blue circle) represent the channel numbers. The localization of CZ position was based on the international 10-20 EEG system.
Measurement Variables
Independent variables
The independent variables for this study will be obtained from the preliminary descriptive analyses on age (young and old), and task (control and four n-back) at five regions of interest (ROI) namely: Dorsolateral Pre-Frontal Cortex (DLPFC), Medial Pre-frontal cortex (MPFC), Supplementary Motor Area (SMA), Primary Motor Cortex (M1), and Somatosensory Association Cortex (SAC).
Dependent variables
The dependent variables will be the behavioral response rates for each n-back task, postural control data (CoP sway and sway velocity), and fNIRS oxygenated concentration values (HbO). The behavioral data will be obtained for the n-back tasks as response time (RT) and accuracy (d’) recorded by E-prime.
The dependent variable for postural control will be the Center of Pressure (CoP) sway measured as 95% ellipse area, and mean velocity computed by the Mobility lab software (APDM, Inc.). Wearable sensors have been widely used for gait, postural control and movement based studies for various populations (Fortaleza et al., 2017; Howell et al., 2017; Pal et al., 2016; Horak et al., 2015). The wearable sensors have shown to be reliable and valid in various studies involving testing for postural control across various populations as well. The recording of the data will be by two wearable sensors. I decided to include these measures since they are widely used to assess postural control during double-leg stance on land (Ruhe et al., 2010), and also while increasing cognitive load in elderly (Moghadam et al., 2011).
The fNIRS system gives raw concentration values for oxygenation and deoxygenation. The oxygenated hemoglobin concentration levels (HbO) for the five ROI’s (DLPFC, MPFC, SMA, M1, and SAC) will be used for this study.
Data analyses
Two sets of data are obtained at the end of the experiment for both groups. (1) CoP sway area (95% ellipse area) and sway velocity (2) fNIRS raw data consisting of HbO for 44 channels. In this section, a detailed explanation of the preprocessing and data analyses for each set of raw data has been discussed.
Behavioral data
The behavioral data for the response rate for each n-back condition will be obtained from E-prime as registered as button clicks and analyzed in Excel. There are two dependent variables: Response Time (RT) and accuracy (represented as d’).
The response time (RT) is calculated as the time taken to respond to each target, which will be recorded in E-prime and later extracted for further analyses. Studies that have used working memory tasks (Sternberg task) have shown increased reaction times with increasing working memory load (Sternberg 1966; Jensen Tesche 2002; Neath, Bireta, & Surprenant, 2003). n-back has been shown to place similar working memory demands. During the n-back task, participants are expected to monitor given information online, update and manipulate remembered information (for a detailed review see Owen et al., 2005). Given such evidence, I expect to see an increase in reaction time with increasing n-back working memory load.
With respect to accuracy, there are two possible responses to any given target: a Hit Rate (H) and a False Alarm Rate (FA). Given a hit rate< 1 and a false alarm rate >0, followed by unit normal assumption, the accuracy (d’) is calculated as the z score of FA minus the H.
d’ = zFA – zH
where zH is the z score in the old distribution having H proportion above it and ZFA is the z score in the new distribution having FA proportion above it. The calculation for d’ is derived from previous studies by Neath & Surprenant (2003).
Postural data
The primary dependent variable for analyzing postural control will be the CoP sway area (95% ellipse area). The CoP sway area has been used widely in previous studies to assess standing postural control or sway on land in healthy old and clinical populations (Gray et al., 2014; Moghadam et al., 2011; Qiu & Xiong, 2015; Ruhe et al., 2010) with high reliability coefficients of 0.86 (Moghadam et al., 2011). Add velocity.
fNIRS data
Once the fNIRS raw data is collected using ETG-4000, offline analyses will be performed using Matlab (The Mathworks, Inc., Natick, Massachusetts, USA). In addition to the fNIRS raw data collected during the experiment, the probe positioning and the head dimensions are obtained using the Polhemus. The Polhemus data is first analyzed to obtain individual brain maps as discussed under the subheading “Registration”. Following this, the fNIRS raw data is preprocessed to obtain the dependent variables as discussed under the section “Preprocessing”.
Registration
ROIs will be selected via Polhemus PATRIOT digitizer channel registration analyses. The ROIs selected for this study are hypotheses driven and are located in the three main cortical lobes namely frontal, motor and parietal. Within these lobes, specific regions will be isolated for the final analyses namely: Dorsolateral Pre-frontal cortex (DLPFC), Medial Pre-frontal cortex (MPFC), Supplementary Motor Area (SMA), Primary Motor Cortex (M1) and Somatosensory Association Cortex (SAC). These regions have been selected based on the neural correlates for postural control (for review refer to (Herold, Wiegel, et al., 2017)) and working memory (for review refer to (Owen et al., 2005b)) tasks. Optode placements in each cap will be located using the Polhemus PATRIOT 3D digitizing system to accurately define the ROIs. After the isolation of the fNIRS data from these ROI, all channels with 50% or greater area overlap within a region will be averaged together based off of MRIcro registration (Rorden & Brett, 2000).
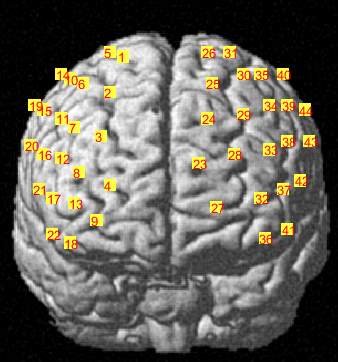
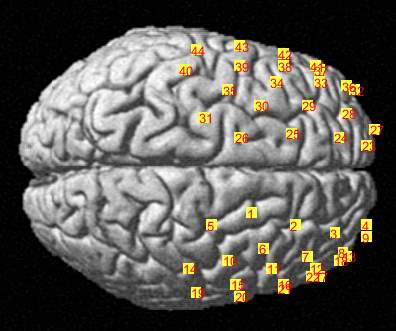

C
Figure 3.6 Brain registration maps illustrating (A) Frontal view of the brain with two probe sets on right and left hemispheres, where the numbers represent the channel numbers, (B) Superior view of the brain with two probe sets on right and left hemispheres, where the numbers represent the channel numbers, (C) Participant head superior view.
fNIRS Data Preprocessing
fNIRS data during a postural control task has been analyzed in various ways in the literature. The raw data will be filtered using wavelet minimum description length (MDL: Gaussian low-pass FWHM at 4s) as per (Brigadoi et al., 2014, 2012). Using NIRS-SPM, the data will be pre-colored and pre-whitened according to (Ye, Tak, Jang, Jung, & Jang, 2009b). A 15s rest ISI prior to the onset of each task will be used as a baseline to remove task-irrelevant noise and signal drift over time (Fu et al., 2015) from the task signal. Finally, each ROI will be represented as one or multiple channels for each participant, with channel selection for each ROI determined by using a >50% channel overlap threshold as reported by the NIRS-SPM registration process (Ye et al., 2009b).
For each participant, the periods of the waveforms will be extracted individually.
The period of the waveforms used in the analyses is also determined for each participant individually. The area under the curve (AUC) will then be calculated by using the period as the starting and ending time points. The period here is time 0 seconds. As each condition is timed for 80 seconds, the AUC end period is at 80 seconds. Using the standard trapezoid function in Matlab, the AUC will be calculated for HbO waveforms. The area under the curve, above and below the baseline will be calculated to conduct statistical analyses.
Statistical Analyses
Descriptive statistics
Descriptive statistics will be obtained from the SPSS statistical software. Normality assumptions for the dependent variables will be checked for between and within groups. Mean and standard deviation values for the independent variables will be reported. Additionally, a preliminary descriptive analysis will be performed to see if there are gender differences. In case I find gender differences, gender will be included as a between-subjects factor.
Preliminary ANOVA will be performed to assess working memory load and neural activation in potential ROIs. Three preliminary ANOVA will be conducted to assess working memory load. Two within-subject ANOVA (collapsed across groups) will assess potential differences in accuracy (d’) and response time (RT) for 0-back, 1-back, 2-back and 3-back tasks. In addition, I will conduct a within-subject preliminary ANOVA to assess potential differences in CoP sway area for control, 0-back, 1-back, 2-back and 3-back tasks. Findings from the main effects and post-hoc analyses will be used to inform the independent variables that will be used for the main analyses. For example, I may find that 0-back and 1-back tasks are of similar levels of difficulty compared to 2-back and 3-back tasks. In that case, I would have 2 levels of working memory load. Or, I may find that 0-back differs from 1- and 2-back, which differ from 3-back, resulting in 3 levels of working memory load.
Additional preliminary analyses will be conducted to assess potential hemispheric differences for the five ROIs (DLPFC, MPFC, SMA, M1, and SAC).
Inferential statistics
Inferential statistics will include three different analyses of dependent variables for the postural control and fNIRS data. These are discussed in detail under individual subheadings below.
1. To what extent does the increase of cognitive load influence postural control in young and old adults?
A Repeated Measures ANOVA will be used to compare young and old groups for the different load conditions (control and four n-back tasks (0-back, 1-back, 2-back, and 3-back)) with age as the between-subjects factor. I will look for significant main effects and interactions for the dependent variable; 95% ellipse area. Mean velocity and range will be explored as well. The assumptions of homoscedasticity (the variance around the regression line is same for all values) and sphericity (a condition where the variances of the differences between all the possible combinations of groups are equal) will be tested using Box’s M and Levine’s Test. The omnibus ANOVA will determine if there are significant differences between the means for the dependent variables. This will help us understand if there are significant differences between the conditions for these variables. Significant effects will be further probed using post-hoc analysis. This could potentially include a significant 2- way (Age x Condition) interaction. I will control for multiple comparisons by using Tukey’s HSD and a significance level of alpha = 0.05. Effect sizes of Cohen’s-d will be computed for the significant effects.
If the data violates the assumptions of homoscedasticity (equal variance) and sphericity (or compound symmetry: all pairs of measurements are equally correlated), a multilevel or marginal framework will be employed instead. This will likely include modeling with Generalized estimating equations (GEE), which will allow for more flexibility in controlling for the correlation between repeated measures. If there is a mild violation of sphericity, the Greenhouse-Geiser correction to degrees of freedom will be used within the repeated measures ANOVA. If there is a larger violation of sphericity, a GEE will be used (Hanley, Negassa, Edwardes, & Forrester, 2003; Schwartz, & Fargo, 2017).
- What is the hemodynamic response in the frontal, motor and parietal cortical regions with increasing cognitive load in young and old adults and how do activations in these regions relate to each other during our tasks?
A Multivariate Approach to Repeated Measures ANOVA will be used to control for correlations between and among the independent and dependent variables. I plan to compare young and old groups on five tasks. Separate analyses will be performed for oxygenated hemoglobin (HbO) beta values which are the primary dependent variables. The between-subjects factor will be Group (Young, Old). The within-subjects factors will be the conditions (control, 0-back, 1-back, 2-back and 3-back), Regions of Interest (ROIs) (DLPFC, MPFC, SMA, M1, and SAC) and Hemisphere (left, right).
To determine whether the fNIRS signal during 0-back, 1-back, 2-back, 3-back and control tasks differed significantly in the ROI’s that are known to be activated during postural control, a two-way within-subjects ANOVAs; one for the HbO Hemodynamic response function (HRF) area under the curve will be performed. The within-subjects factors for both analyses will be condition, with five levels (0-back, 1-back, 2-back, 3-back, and control) and ROI, with five levels: DLPFC, MPFC, SMA, M1, and SAC. All main effects and interactions will be tested using the Greenhouse-Geisser correction for potential violations of the sphericity assumption. Post-hoc comparisons (Tukey HSD) and a significance level of alpha = 0.05 will be conducted with pairwise tests of simple main effects and paired sample t-tests for interactions. I will also look at significant main effects in the HbO beta values. Post-hoc analyses will be performed to see individual differences. For each group, I will additionally look for a 3-way (Age x Condition x ROI) interaction.
If the data violates the assumptions of homoscedasticity (equal variance) and sphericity (or compound symmetry: all pairs of measurements are equally correlated), a multilevel or marginal framework will be employed instead. This will likely include modeling with Generalized estimating equations (GEE), which will allow for more flexibility in controlling for the correlation between repeated measures. If there is a mild violation of sphericity, the Greenhouse-Geiser correction to degrees of freedom will be used within the repeated measures ANOVA. If there is a larger violation of sphericity, a GEE will be used (Hanley et al., 2003; Schwartz, & Fargo, 2017).
In addition to this, a functional connectivity analysis using Granger Causality to determine the causal relationships between ROI according to transfer entropy (Seth, 2010; Seth, Barrett, & Barnett, 2015). In Granger Causality, the signal from one ROI is used in a statistical model to predict the subsequent signal in another ROI, showing “information flow” or connectivity between two regions, as opposed to differences between regions. Time-domain functional connectivity will be processed using the Multivariate Granger Causality toolbox (Barnett & Seth, 2011, 2014) in order to establish whether there were causal pathways across the five ROI’s associated with postural control and working memory. Granger Causality in this study is the measurement of information flow or transfer from one ROI to another ROI, also referred to as “transfer entropy” (Barnett, Barrett, & Seth, 2009) while they are performing specific tasks. This will be measured by a log likelihood F, in which the full model will include the past hemodynamic information of one ROI regressed onto the past hemodynamic response of a second ROI. To account for multiple comparisons, significance values (p-values) will be corrected using false discovery rate.
Additionally, Granger Causality analysis will help in explaining the differences in the significant connectomes between groups (young vs. old) and between tasks (seated rest, 0-back, 2-back, 3-back). This information will be useful for explicating the nature of neurocognitive differences between the groups (Battaglia et al., 2017; Gao et al., 2016; Plichta et al., 2006; Xu et al., 2015)
- What is the relationship between the activation levels of frontal, motor and parietal cortical regions and the static postural control measures in young and old adults?
A multivariate regression will be used to understand the significant relationship between the average area under the curve values of HbO for five regions and postural control measures: CoP sway area and sway velocity during the n-back tasks for the two groups (Green & Salkind, 2017; Plichta et al., 2006).
Limitations
Following are possible limitations of this study:
- fNIRS cap limitations: the ETG-4000 system allows for the measurement of up to 48 channels on an individual’s scalp. Therefore, this study will focus on the bilateral activation in the frontal, motor and parietal, left and right cortices.
- Another major limitation of this study would be using just a static postural control task and not including a dynamic postural control task. The movement involved in dynamic postural control task is a challenge due to data interpretation due to added noise. This could potentially make the data hard to interpret and unusable (Goh et al., 2017). Other studies have also reported the motion artifacts involved with the fNIRS tool and the variety of data corrections (Brigadoi et al., 2014; Goh et al., 2017) that need to be considered. Given such evidence and to avoid unnecessary noise, a dynamic postural control task will not be used for the current study.
- Another limitation of this study is testing for other cognitively demanding tasks. Due to the nature of the design and the duration of the study, only an n-back task will be used to test the working memory paradigm. n-back has been extensively used to test the working memory paradigms (Owen et al., 2005b). Additionally, n-back allows to control for cognitive demands and modify it in the design by increasing the cognitive load from 0-back all the way to 3-back or more. Future studies with other cognitively demanding tasks can be designed to test other similar tasks that change the cognitive load and affect postural control.
- Physiological arousal is a limitation of the current study. Previous research by Maki and McIlroy have shown that the nature of the secondary task in a dual-task paradigm may result in unwanted arousal. This unexpected arousal is a potential confounder while understanding the influence of attention on postural control (Maki & Mcilroy, 1996). Similarly, this study may see such confounding factors if arousal were to be shown in certain individuals. The way to measure arousal would be using skin conductance equipment. The current study will not use any additional measures to control for any unexpected arousal.
References
Alexander, B. H., Rivara, F. P., & Wolf, M. E. (1992). The cost and frequency of hospitalization for fall-related injuries in older adults. American Journal of Public Health, 82(7), 1020–1023. https://doi.org/10.2105/AJPH.82.7.1020
Aziz, O., Russell, C. M., Park, E. J., & Robinovitch, S. N. (2014). The effect of window size and lead time on pre-impact fall detection accuracy using support vector machine analysis of waist mounted inertial sensor data. In 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (pp. 30–33). IEEE. https://doi.org/10.1109/EMBC.2014.6943521
Baddeley, A. D., & Hitch, G. (1974). Working memory. The Psychology of Learning and Motivation Advances in Research and Theory. https://doi.org/10.1016/S0079-7421(08)60452-1
Baddeley, A., Logie, R., Bressi, S., Sala, S. Della, & Spinnler, H. (1986). Dementia and Working Memory. The Quarterly Journal of Experimental Psychology Section A, 38(4), 603–618. https://doi.org/10.1080/14640748608401616
Bandettini, P. (2007). Functional MRI today. International Journal of Psychophysiology, 63(2), 138–145. https://doi.org/10.1016/j.ijpsycho.2006.03.016
Barnett, L., Barrett, A. B., & Seth, A. K. (2009). Granger Causality and Transfer Entropy Are Equivalent for Gaussian Variables. Physical Review Letters, 103(23), 238701. https://doi.org/10.1103/PhysRevLett.103.238701
Barnett, L., & Seth, A. K. (2011). Behaviour of Granger causality under filtering: Theoretical invariance and practical application. Journal of Neuroscience Methods, 201(2), 404–419. https://doi.org/10.1016/j.jneumeth.2011.08.010
Barnett, L., & Seth, A. K. (2014). The MVGC multivariate Granger causality toolbox: A new approach to Granger-causal inference. Journal of Neuroscience Methods, 223, 50–68. https://doi.org/10.1016/j.jneumeth.2013.10.018
Barros de Oliveira, C., Torres de Medeiros, Í. R., Frota, N. A. F., Greters, M. E., & Conforto, A. B. (2008). Balance control in hemiparetic stroke patients: Main tools for evaluation. The Journal of Rehabilitation Research and Development, 45(8), 1215. https://doi.org/10.1682/JRRD.2007.09.0150
Barth, B., Strehl, U., Fallgatter, A. J., & Ehlis, A.-C. (2016). Near-Infrared Spectroscopy based Neurofeedback of Prefrontal Cortex Activity: A Proof-of-Concept Study. Frontiers in Human Neuroscience. Frontiers Media SA. https://doi.org/10.3389/fnhum.2016.00633
Battaglia, D., Boudou, T., A Hansen, E. C., Chettouf, S., Daffertshofer, A., Randal McIntosh, A., … Randal McIntosh, vunl A. (2017). Functional Connectivity Dynamics of the Resting State across the Human Adult Lifespan. https://doi.org/10.1101/107243
Boisgontier, M. P., Beets, I. A. M., Duysens, J., Nieuwboer, A., Krampe, R. T., & Swinnen, S. P. (2013). Age-related differences in attentional cost associated with postural dual tasks: Increased recruitment of generic cognitive resources in older adults. Neuroscience & Biobehavioral Reviews, 37(8), 1824–1837. https://doi.org/10.1016/j.neubiorev.2013.07.014
Bolton, D. A. E. (2015). The role of the cerebral cortex in postural responses to externally induced perturbations. Neuroscience and Biobehavioral Reviews, 57, 142–155. https://doi.org/10.1016/j.neubiorev.2015.08.014
Boschin, E. A., & Buckley, M. J. (2015). Differential contributions of dorsolateral and frontopolar cortices to working memory processes in the primate. Frontiers in Systems Neuroscience, 9, 144. https://doi.org/10.3389/fnsys.2015.00144
Braver, T. S., Cohen, J. D., Nystrom, L. E., Jonides, J., Smith, E. E., & Noll, D. C. (1997). A parametric study of prefrontal cortex involvement in human working memory. NeuroImage, 5(1), 49–62. https://doi.org/10.1006/nimg.1996.0247
Brigadoi, S., Ceccherini, L., Cutini, S., Scarpa, F., Scatturin, P., Selb, J., … Cooper, R. J. (2014). Motion artifacts in functional near-infrared spectroscopy: A comparison of motion correction techniques applied to real cognitive data. NeuroImage, 85, 181–191. https://doi.org/10.1016/j.neuroimage.2013.04.082
Brigadoi, S., Cutini, S., Scarpa, F., Scatturin, P., & Dell’Acqua, R. (2012). Exploring the role of primary and supplementary motor areas in simple motor tasks with fNIRS. Cognitive Processing, 13(S1), 97–101. https://doi.org/10.1007/s10339-012-0446-z
Caldas, R., Mundt, M., Potthast, W., Buarque de Lima Neto, F., & Markert, B. (2017). A systematic review of gait analysis methods based on inertial sensors and adaptive algorithms. Gait & Posture, 57(June), 204–210. https://doi.org/10.1016/j.gaitpost.2017.06.019
Camicioli, R., Howieson, D., Lehman, S., & Kaye, J. (1997). Talking while walking: the effect of a dual task in aging and Alzheimer’s disease. Neurology, 48(4), 955–958. https://doi.org/10.1212/WNL.48.4.955
Campbell, A. J., Borrie, M. J., & Spears, G. F. (1989). Risk Factors for Falls in a Community-Based Prospective Study of People 70 Years and Older. Journal of Gerontology, 44(5), M112–M117. https://doi.org/10.1093/geronj/44.5.M112
Canadian Society for Exercise Physiology. (2002). PAR-Q and you, (revised), 1.
Carter, N. D., Kannus, P., & Khan, K. (2012). Exercise in the Prevention of Falls in Older People. Sports Medicine, 31(6), 427–438. https://doi.org/10.2165/00007256-200131060-00003
Carter, N. D., Kannus, P., Khan, K., Polskaia, N., Richer, N., Dionne, E., … Carter, C. S. (2017). Mental workload during n-back task—quantified in the prefrontal cortex using fNIRS. NeuroImage, 9(1), 1–10. https://doi.org/10.1093/ageing/afl077
CDC. (2016). Center For Disease Control and Prevention. Retrieved from https://www.cdc.gov/homeandrecreationalsafety/falls/
CDC. (2017). Centers for Disease Control and Prevention. Retrieved from https://www.cdc.gov/steadi/
Chance, B., Anday, E., Nioka, S., Zhou, S., Hong, L., Worden, K., … Thomas, R. (1998). A novel method for fast imaging of brain function, non-invasively, with light. Optics Express, 2(10), 411. https://doi.org/10.1364/OE.2.000411
Chang, J. T., Morton, S. C., Rubenstein, L. Z., Mojica, W. A., Maglione, M., Suttorp, M. J., … Shekelle, P. G. (2004). Interventions for the prevention of falls in older adults: systematic review and meta-analysis of randomised clinical trials, 328(March), 1–7.
Chein, J. M., & Fiez, J. A. (2010). Evaluating models of working memory through the effects of concurrent irrelevant information. Journal of Experimental Psychology: General, 139(1), 117–137. https://doi.org/10.1037/a0018200
Crosson, B., Ford, A., McGregor, K. M., Meinzer, M., Cheshkov, S., Li, X., … Briggs, R. W. (2010). Functional imaging and related techniques: an introduction for rehabilitation researchers. Journal of Rehabilitation Research and Development, 47(2), vii–xxxiv. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/20593321
Culham, J. C., & Valyear, K. F. (2006). Human parietal cortex in action. Current Opinion in Neurobiology, 16(2), 205–212. https://doi.org/10.1016/j.conb.2006.03.005
Dale, A. M., & Buckner, R. L. (1997). Selective averaging of rapidly presented individual trials using fMRI. Human Brain Mapping, 5(5), 329–340. https://doi.org/10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5
Delpy, D. T., & Cope, M. (1997). Quantification in tissue near–infrared spectroscopy. Philosophical Transactions of the Royal Society B: Biological Sciences, 352(1354).
Demitri, M. (2013). Types of Brain Imaging Techniques | Psych Central. Retrieved October 2, 2017, from http://psychcentral.com/lib/types-of-brain-imaging-techniques/
Dieler, A. C., Tupak, S. V., & Fallgatter, A. J. (2012). Functional near-infrared spectroscopy for the assessment of speech related tasks. Brain and Language, 121(2), 90–109. https://doi.org/10.1016/j.bandl.2011.03.005
Dommer, L., Jäger, N., Scholkmann, F., Wolf, M., & Holper, L. (2012). Between-brain coherence during joint n-back task performance: A two-person functional near-infrared spectroscopy study. Behavioural Brain Research, 234(2), 212–222. https://doi.org/10.1016/j.bbr.2012.06.024
Dunn, J. E., Rudberg, M. A., Furner, S. E., & Cassel, C. K. (1992). Mortality, disability, and falls in older persons: The role of underlying disease and disability. American Journal of Public Health, 82(3), 395–400. https://doi.org/10.2105/AJPH.82.3.395
El-Gohary, M., Peterson, D., Gera, G., Horak, F. B., & Huisinga, J. M. (2017). Validity of the Instrumented Push and Release Test to Quantify Postural Responses in Persons With Multiple Sclerosis. Archives of Physical Medicine and Rehabilitation, 98(7), 1325–1331. https://doi.org/10.1016/J.APMR.2017.01.030
Erdfelder, E., Faul, F., Buchner, A., & Lang, A. G. (2009). Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods, 41(4), 1149–1160. https://doi.org/10.3758/BRM.41.4.1149
Faul, F., Erdfelder, E., Lang, A.-G., & Buchner, A. (2007). G*Power: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. https://doi.org/10.3758/BF03193146
Fernie, G. R., Gryfe, C. I., Holliday, P. J., & Llewellyn, A. (1982). THE RELATIONSHIP OF POSTURAL SWAY IN STANDING TO THE INCIDENCE OF FALLS IN GERIATRIC SUBJECTS. Age and Ageing, 11(1), 11–16. https://doi.org/10.1093/ageing/11.1.11
Ferrari, M., Bisconti, S., Spezialetti, M., Moro, S. B., Di Palo, C., Placidi, G., & Quaresima, V. (2014). Prefrontal cortex activated bilaterally by a tilt board balance task: A functional near-infrared spectroscopy study in a semi-immersive virtual reality environment. Brain Topography, 27(3), 353–365. https://doi.org/10.1007/s10548-013-0320-z
Ferrari, M., & Quaresima, V. (2012). A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. NeuroImage, 63(2), 921–935. https://doi.org/10.1016/j.neuroimage.2012.03.049
Fregni, F., Boggio, P. S., Nitsche, M., Bermpohl, F., Antal, A., Feredoes, E., … Pascual-Leone, A. (2005). Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Experimental Brain Research, 166(1), 23–30. https://doi.org/10.1007/s00221-005-2334-6
Fujimoto, H., Mihara, M., Hattori, N., Hatakenaka, M., Kawano, T., Yagura, H., … Mochizuki, H. (2014). Cortical changes underlying balance recovery in patients with hemiplegic stroke. NeuroImage, 85, 547–554. https://doi.org/10.1016/j.neuroimage.2013.05.014
Fujita, H., Kasubuchi, K., Wakata, S., Hiyamizu, M., & Morioka, S. (2016a). 2015 – Role of the Frontal Cortex in Standing Postural Sway Tasks While Dual-Tasking A Functional Near-Infrared Spectroscopy Study Examining Working Memory Capacity. BioMed Research International, 2016, 10. https://doi.org/http://dx.doi.org/10.1155/2016/7053867
Fujita, H., Kasubuchi, K., Wakata, S., Hiyamizu, M., & Morioka, S. (2016b). Role of the Frontal Cortex in Standing Postural Sway Tasks While Dual-Tasking: A Functional Near-Infrared Spectroscopy Study Examining Working Memory Capacity. BioMed Research International, 2016, 1–10. https://doi.org/10.1155/2016/7053867
Fukuyama, H., Ouchi, Y., Matsuzaki, S., & Nagahama, Y. (1997). Brain functional activity during gait in normal subjects : a SPECT study, 228, 183–186.
Gao, L., Wu, T., Jankovic, J., DeLong, M., Wichmann, T., Fox, M., … Chan, P. (2016). The study of brain functional connectivity in Parkinson’s disease. Translational Neurodegeneration, 5(1), 18. https://doi.org/10.1186/s40035-016-0066-0
Goh, K. L., Morris, S., Lee, W. L., Ring, A., Tan, T., Mehagnoul-Schipper, D. J., … Sakoda, S. (2017). Motion artifacts in functional near-infrared spectroscopy: A comparison of motion correction techniques applied to real cognitive data. NeuroImage, 85(2), 1–10. https://doi.org/10.1016/j.neuroimage.2013.04.082
Goldman-Rakic, P. S. (2011). Circuitry of Primate Prefrontal Cortex and Regulation of Behavior by Representational Memory. In Comprehensive Physiology. Hoboken, NJ, USA: John Wiley & Sons, Inc. https://doi.org/10.1002/cphy.cp010509
Gordon, E. M., Breeden, A. L., Bean, S. E., & Vaidya, C. J. (2014). Working memory-related changes in functional connectivity persist beyond task disengagement. Human Brain Mapping, 35(3), 1004–1017. https://doi.org/10.1002/hbm.22230
Gray, V. L., Ivanova, T. D., & Garland, S. J. (2014). Reliability of center of pressure measures within and between sessions in individuals post-stroke and healthy controls. Gait and Posture, 40(1), 198–203. https://doi.org/10.1016/j.gaitpost.2014.03.191
Green, S., & Salkind, N. (2017). Using SPSS for Windows and Macintosh. (8th Edition, Ed.). Pearson. Retrieved from https://www.pearson.com/us/higher-education/program/Green-Using-SPSS-for-Windows-and-Macintosh-Books-a-la-Carte-8th-Edition/PGM14536.html
Hanley, J. A., Negassa, A., Edwardes, M. D. deB., & Forrester, J. E. (2003). Statistical Analysis of Correlated Data Using Generalized Estimating Equations: An Orientation. American Journal of Epidemiology, 157(4), 364–375. https://doi.org/10.1093/aje/kwf215
Herff, C., Heger, D., Fortmann, O., Hennrich, J., Putze, F., & Schultz, T. (2014a). Mental workload during n-back task—quantified in the prefrontal cortex using fNIRS. Frontiers in Human Neuroscience, 7, 935. https://doi.org/10.3389/fnhum.2013.00935
Herff, C., Heger, D., Fortmann, O., Hennrich, J., Putze, F., & Schultz, T. (2014b). Mental workload during n-back task—quantified in the prefrontal cortex using fNIRS. Frontiers in Human Neuroscience, 7, 935. https://doi.org/10.3389/fnhum.2013.00935
Herold, F., Orlowski, K., Börmel, S., & Müller, N. G. (2017). Cortical activation during balancing on a balance board. Human Movement Science, 51, 51–58. https://doi.org/10.1016/j.humov.2016.11.002
Herold, F., Wiegel, P., Scholkmann, F., Thiers, A., Hamacher, D., & Schega, L. (2017). Functional near-infrared spectroscopy in movement science: a systematic review on cortical activity in postural and walking tasks. Neurophotonics, 4(4), 41403. https://doi.org/10.1117/1.NPh.4.4.041403
Holtzer, R., Epstein, N., Mahoney, J. R., Izzetoglu, M., & Blumen, H. M. (2014a). Neuroimaging of mobility in aging: A targeted review. Journals of Gerontology – Series A Biological Sciences and Medical Sciences, 69(11), 1375–1388. https://doi.org/10.1093/gerona/glu052
Holtzer, R., Epstein, N., Mahoney, J. R., Izzetoglu, M., & Blumen, H. M. (2014b, August 20). Neuroimaging of mobility in aging: A targeted review. Journals of Gerontology – Series A Biological Sciences and Medical Sciences. Oxford University Press.
Holtzer, R., Mahoney, J. R., Izzetoglu, M., Izzetoglu, K., Onaral, B., & Verghese, J. (2011). fNIRS study of walking and walking while talking in young and old individuals. Journals of Gerontology – Series A Biological Sciences and Medical Sciences, 66 A(8), 879–887. https://doi.org/10.1093/gerona/glr068
Holtzer, R., Wang, C., & Verghese, J. (2012). The relationship between attention and gait in aging: facts and fallacies. Motor Control, 16(1), 64–80. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22402221
Horak, F. B. (2006). Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age and Ageing, 35(SUPPL.2), 7–11.
Horak, F. B., & Mancini, M. (2013). Objective biomarkers of balance and gait for Parkinson’s disease using body-worn sensors. Movement Disorders. https://doi.org/10.1002/mds.25684
Horak, F. B., Shupert, C. L., & Mirka, A. (1989). Components of postural dyscontrol in the elderly: A review. Neurobiology of Aging, 10(6), 727–738. https://doi.org/10.1016/0197-4580(89)90010-9
Horak, F., King, L., & Mancini, M. (2015). Role of Body-Worn Movement Monitor Technology for Balance and Gait Rehabilitation. Physical Therapy, 95(3), 461–470. https://doi.org/10.2522/ptj.20140253
Huang, D., Mao, Y., Chen, P., & Li, L. (2014). Virtual reality training improves balance function. Neural Regeneration Research, 9(17), 1628. https://doi.org/10.4103/1673-5374.141795
Huang, H. J., & Mercer, V. S. (2001). Dual-task methodology: applications in studies of cognitive and motor performance in adults and children. Pediatric Physical Therapy : The Official Publication of the Section on Pediatrics of the American Physical Therapy Association, 13(3), 133–140. https://doi.org/10.1097/00001577-200110000-00005
Huxhold, O., Li, S.-C., Schmiedek, F., & Lindenberger, U. (2006). Dual-tasking postural control: Aging and the effects of cognitive demand in conjunction with focus of attention. Brain Research Bulletin, 69(3), 294–305. https://doi.org/10.1016/j.brainresbull.2006.01.002
Jacobs, J. V., & Horak, F. B. (2007). Cortical control of postural responses. Journal of Neural Transmission, 114(10), 1339–1348. https://doi.org/10.1007/s00702-007-0657-0
Jaeggi, S. M., Seewer, R., Nirkko, A. C., Eckstein, D., Schroth, G., Groner, R., & Gutbrod, K. (2003). Does excessive memory load attenuate activation in the prefrontal cortex? Load-dependent processing in single and dual tasks: Functional magnetic resonance imaging study. NeuroImage, 19(2), 210–225. https://doi.org/10.1016/S1053-8119(03)00098-3
Jensen, O., & Tesche, C. D. (2002). Frontal theta activity in humans increases with memory load in a working memory task. European Journal of Neuroscience, 15, 1395–1399. Retrieved from http://ojensen.ruhosting.nl/jensen02c.pdf
Kane, M. J. (2002). Full-Text, 9(4), 637–671. https://doi.org/10.3758/BF03196323
King, L. A., & Horak, F. B. (2009). Delaying Mobility Disability in People With Parkinson Disease Using a Sensorimotor Agility Exercise Program. Physical Therapy, 89(4), 384–393. https://doi.org/10.2522/ptj.20080214
Kober, S. E., Kurzmann, J., & Neuper, C. (2012). Cortical correlate of spatial presence in 2D and 3D interactive virtual reality: An EEG study. International Journal of Psychophysiology, 83(3), 365–374. https://doi.org/10.1016/j.ijpsycho.2011.12.003
Koenraadt, K. L. M., Duysens, J., Meddeler, B. M., & Keijsers, N. L. W. (2013). Hand tapping at mixed frequencies requires more motor cortex activity compared to single frequencies: An fNIRS study. Experimental Brain Research, 231(2), 231–237. https://doi.org/10.1007/s00221-013-3686-y
Koenraadt, K. L. M., Roelofsen, E. G. J., Duysens, J., & Keijsers, N. L. W. (2014a). Cortical control of normal gait and precision stepping: An fNIRS study. NeuroImage, 85. https://doi.org/10.1016/j.neuroimage.2013.04.070
Koenraadt, K. L. M., Roelofsen, E. G. J., Duysens, J., & Keijsers, N. L. W. (2014b). Cortical control of normal gait and precision stepping: An fNIRS study. NeuroImage, 85, 415–422. https://doi.org/10.1016/j.neuroimage.2013.04.070
Krishnan, V., Cho, Y., & Mohamed, O. (2017). Role of impaired vision during dual-task walking in young and older adults. Gait and Posture, 57(August 2016), 136–140. https://doi.org/10.1016/j.gaitpost.2017.06.006
Lacour, M., Bernard-Demanze, L., & Dumitrescu, M. (2008). Posture control, aging, and attention resources: Models and posture-analysis methods. Neurophysiologie Clinique, 38(6), 411–421. https://doi.org/10.1016/j.neucli.2008.09.005
Lajoie, Y., & Gallagher, S. . (2004). Predicting falls within the elderly community: comparison of postural sway, reaction time, the Berg balance scale and the Activities-specific Balance Confidence (ABC) scale for comparing fallers and non-fallers. Archives of Gerontology and Geriatrics, 38(1), 11–26. https://doi.org/10.1016/S0167-4943(03)00082-7
Leff, D. R., Orihuela-Espina, F., Elwell, C. E., Athanasiou, T., Delpy, D. T., Darzi, A. W., & Yang, G.-Z. (2011). Assessment of the cerebral cortex during motor task behaviours in adults: A systematic review of functional near infrared spectroscopy (fNIRS) studies. NeuroImage, 54(4), 2922–2936. https://doi.org/10.1016/j.neuroimage.2010.10.058
Li, K. Z. H., Roudaia, E., Lussier, M., Bherer, L., Leroux, A., & McKinley, P. A. (2010). Benefits of Cognitive Dual-Task Training on Balance Performance in Healthy Older Adults. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 65A(12), 1344–1352. https://doi.org/10.1093/gerona/glq151
Lipsitz, L. A., Jonsson, P. V, Kelley, M. M., & Koestner, J. S. (1991). Causes and Correlates of Recurrent Falls in Ambulatory Frail Elderly. Journal of Gerontology: MEDICAL SCIENCES, 46(4), 14–122. https://doi.org/10.1093/geronj/46.4.M114
Litvan, I., Mohr, E., Williams, J., Gomez, C., & Chase, T. N. (1991). Differential memory and executive functions in demented patients with Parkinson’s and Alzheimer’s disease. Journal of Neurology, Neurosurgery & Psychiatry, 54(1), 25–29. https://doi.org/10.1136/jnnp.54.1.25
Maccotta, L., Zacks, J. M., & Buckner, R. L. (2001). Rapid Self-Paced Event-Related Functional MRI: Feasibility and Implications of Stimulus- versus Response-Locked Timing. NeuroImage, 14(5), 1105–1121. https://doi.org/10.1006/nimg.2001.0912
Magaziner, J., Simonsick, E. M., Kashner, T. M., Hebel, J. R., & Kenzora, J. E. (1989). Survival experience of aged hip fracture patients. American Journal of Public Health, 79(3), 274–278. https://doi.org/10.2105/AJPH.79.3.274
Maidan, I., Bernad-Elazari, H., Gazit, E., Giladi, N., Hausdorff, J. M., & Mirelman, A. (2015). Changes in oxygenated hemoglobin link freezing of gait to frontal activation in patients with Parkinson disease: an fNIRS study of transient motor-cognitive failures. Journal of Neurology, 262(4), 899–908. https://doi.org/10.1007/s00415-015-7650-6
Maki, B. E., & Mcilroy, W. E. (1996). Influence of Arousal and Attention on the Control of Postural Sway. Journal of Vestibular Research, 6(I), 53–59. https://doi.org/0957427195000143 [pii]
Man, M. Y., Ong, M. S., Mohamad, M. S., Deris, S., Sulong, G., Yunus, J., & Che Harun, F. K. (2015). A Review on the Bioinformatics Tools for Neuroimaging. The Malaysian Journal of Medical Sciences : MJMS, 22(Spec Issue), 9–19. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/27006633
Mancini, M., & Horak, F. B. (2010a). The relevance of clinical balance assessment tools to differentiate balance deficits. European Journal of Physical and Rehabilitation Medicine, 46(2), 239–248. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/20485226
Mancini, M., & Horak, F. B. (2010b). The relevance of clinical balance assessment tools to differentiate balance deficits. European Journal of Physical and Rehabilitation Medicine, 46(2), 239–248. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/20485226
Mancini, M., King, L., Salarian, A., Holmstrom, L., McNames, J., & Horak, F. B. (2011). Mobility Lab to Assess Balance and Gait with Synchronized Body-worn Sensors. Journal of Bioengineering & Biomedical Science, Suppl 1, 7. https://doi.org/10.4172/2155-9538.S1-007
Manning, D. P., Ayers, I., Jones, C., Bruce, M., & Cohen, K. (1988). The incidence of underfoot accidents during 1985 in a working population of 10,000 Merseyside people. Journal of Occupational Accidents, 10(2), 121–130. https://doi.org/10.1016/0376-6349(88)90026-0
Mansouri, F. A., Rosa, M. G. P., & Atapour, N. (2015). Working Memory in the Service of Executive Control Functions. Frontiers in Systems Neuroscience, 9. https://doi.org/10.3389/fnsys.2015.00166
Martori, A. L. (2013). A Wearable Motion Analysis System to Evaluate Gait Deviations. Retrieved from http://scholarcommons.usf.edu/etd
McKendrick, R., Parasuraman, R., & Ayaz, H. (2015). Wearable functional near infrared spectroscopy (fNIRS) and transcranial direct current stimulation (tDCS): expanding vistas for neurocognitive augmentation. Frontiers in Systems Neuroscience, 9(March), 27. https://doi.org/10.3389/fnsys.2015.00027
Mckeon, P. O., Ingersoll, C. D., Kerrigan, D. C., Saliba, E., Bennett, B. C., & Hertel, J. (2008). Balance training improves function and postural control in those with chronic ankle instability. Medicine and Science in Sports and Exercise, 40(10), 1810–1819. https://doi.org/10.1249/MSS.0b013e31817e0f92
Mihara, M., Miyai, I., Hatakenaka, M., Kubota, K., & Sakoda, S. (2008). Role of the prefrontal cortex in human balance control. NeuroImage, 43(2), 329–336. https://doi.org/10.1016/j.neuroimage.2008.07.029
Miller, K. M., Price, C. C., Okun, M. S., Montijo, H., & Bowers, D. (2009). Is the N-Back Task a Valid Neuropsychological Measure for Assessing Working Memory? Archives of Clinical Neuropsychology, 24(7), 711–717. https://doi.org/10.1093/arclin/acp063
Mirelman, A., Maidan, I., Bernad-Elazari, H., Shustack, S., Giladi, N., & Hausdorff, J. M. (2017). Effects of aging on prefrontal brain activation during challenging walking conditions. Brain and Cognition, 115, 41–46. https://doi.org/10.1016/j.bandc.2017.04.002
Moghadam, M., Ashayeri, H., Salavati, M., Sarafzadeh, J., Taghipoor, K. D., Saeedi, A., & Salehi, R. (2011). Reliability of center of pressure measures of postural stability in healthy older adults: Effects of postural task difficulty and cognitive load. Gait & Posture, 33(4), 651–655. https://doi.org/10.1016/j.gaitpost.2011.02.016
Neath, I., Bireta, T. J., & Surprenant, A. M. (2003). The time-based word length effect and stimulus set specificity. Psychonomic Bulletin & Review, 10(2), 430–434. https://doi.org/10.3758/BF03193866
Nielsen, J. B. (2003). How we walk: central control of muscle activity during human walking. The Neuroscientist : A Review Journal Bringing Neurobiology, Neurology and Psychiatry, 9(3), 195–204. https://doi.org/10.1177/1073858403251978
Overstall, P. W., Exton-Smith, A. N., Imms, F. J., & Johnson, A. L. (1977). Falls in the elderly related to postural imbalance. British Medical Journal, 1(6056), 261–264. https://doi.org/10.1136/BMJ.1.6056.261
Owen, A. M., McMillan, K. M., Laird, A. R., & Bullmore, E. (2005a). N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Human Brain Mapping, 25(1), 46–59. https://doi.org/10.1002/hbm.20131
Owen, A. M., McMillan, K. M., Laird, A. R., & Bullmore, E. (2005b). N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Human Brain Mapping, 25(1), 46–59. https://doi.org/10.1002/hbm.20131
Pellecchia, G. L. (2003a). Postural sway increases with attentional demands of concurrent cognitive task. Gait and Posture, 18(1), 29–34. https://doi.org/10.1016/S0966-6362(02)00138-8
Pellecchia, G. L. (2003b). Postural sway increases with attentional demands of concurrent cognitive task. Gait and Posture, 18(1), 29–34. https://doi.org/10.1016/S0966-6362(02)00138-8
Piirtola, M., & Era, P. (2006). Force platform measurements as predictors of falls among older people – A review. Gerontology, 52(1), 1–16. https://doi.org/10.1159/000089820
Plichta, M. M., Herrmann, M. J., Baehne, C. G., Ehlis, A.-C., Richter, M. M., Pauli, P., & Fallgatter, A. J. (2006). Event-related functional near-infrared spectroscopy (fNIRS): Are the measurements reliable? NeuroImage, 31(1), 116–124. https://doi.org/10.1016/j.neuroimage.2005.12.008
Qiu, H., & Xiong, S. (2015). Center-of-pressure based postural sway measures: Reliability and ability to distinguish between age, fear of falling and fall history. International Journal of Industrial Ergonomics, 47, 28–35. https://doi.org/10.1016/j.ergon.2015.02.004
Rao, A. K., Uddin, J., Gillman, A., & Louis, E. D. (2013). Cognitive motor interference during dual-task gait in essential tremor. Gait and Posture, 38(3), 403–409. https://doi.org/10.1016/j.gaitpost.2013.01.006
Ridderinkhof, K. R., van den Wildenberg, W. P. M., Segalowitz, S. J., & Carter, C. S. (2004). Neurocognitive mechanisms of cognitive control: The role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain and Cognition, 56(2), 129–140. https://doi.org/10.1016/j.bandc.2004.09.016
Rigsby, M. T., & Bigelow, K. E. (1989). Validation of a Commercial Wearable Sensor System for Accurately Measuring Gait on Uneven Terrain. Journal of Chemical Information and Modeling, 53, 160. https://doi.org/10.1017/CBO9781107415324.004
Riley, M. A., Baker, A. A., & Schmit, J. M. (2003). Inverse relation between postural variability and difficulty of a concurrent short-term memory task. Brain Research Bulletin, 62(3), 191–195. https://doi.org/10.1016/j.brainresbull.2003.09.012
Riva, F. (n.d.). Methods for the Quantification of Motor Stability for the.
Roalf, D. R., Moberg, P. J., Xie, S. X., Wolk, D. A., Moelter, S. T., & Arnold, S. E. (2013). Comparative accuracies of two common screening instruments for?classification of Alzheimer’s disease, mild cognitive impairment, and?healthy aging. Alzheimer’s & Dementia, 9(5), 529–537. https://doi.org/10.1016/j.jalz.2012.10.001
Roberts, A. C., Robbins, T. W., & Weiskrantz, L. (1998). The Prefrontal CortexExecutive and Cognitive Functions. Oxford University Press. https://doi.org/10.1093/acprof:oso/9780198524410.001.0001
Rorden, C., & Brett, M. (2000). Stereotaxic Display of Brain Lesions. Behavioural Neurology, 12(4), 191–200. https://doi.org/10.1155/2000/421719
Rosso, A. L., Cenciarini, M., Sparto, P. J., Loughlin, P. J., Furman, J. M., & Huppert, T. J. (2017). Neuroimaging of an attention demanding dual-task during dynamic postural control. Gait & Posture, 57, 193–198. https://doi.org/10.1016/j.gaitpost.2017.06.013
Rubenstein, L. Z. (2006). Falls in older people: Epidemiology, risk factors and strategies for prevention. Age and Ageing, 35(SUPPL.2), ii37-ii41. https://doi.org/10.1093/ageing/afl084
Ruhe, A., Fejer, R., & Walker, B. (2010). The test-retest reliability of centre of pressure measures in bipedal static task conditions – A systematic review of the literature. Gait and Posture, 32(4), 436–445. https://doi.org/10.1016/j.gaitpost.2010.09.012
Rypma, B., Berger, J. S., & D’Esposito, M. (2002). The Influence of Working-Memory Demand and Subject Performance on Prefrontal Cortical Activity. Journal of Cognitive Neuroscience, 14(5), 721–731. https://doi.org/10.1162/08989290260138627
Sankarpandi, S. K., Baldwin, A. J., Ray, J., & Mazzà, C. (2017a). Reliability of inertial sensors in the assessment of patients with vestibular disorders: a feasibility study. BMC Ear, Nose and Throat Disorders, 17(1), 1. https://doi.org/10.1186/s12901-017-0034-z
Sankarpandi, S. K., Baldwin, A. J., Ray, J., & Mazzà, C. (2017b). Reliability of inertial sensors in the assessment of patients with vestibular disorders: a feasibility study. BMC Ear, Nose and Throat Disorders, 17(1), 1. https://doi.org/10.1186/s12901-017-0034-z
Santos, D. A., & Duarte, M. (2016). A public data set of human balance evaluations. PeerJ, 4, e2648. https://doi.org/10.7717/peerj.2648
Sarter, M., Givens, B., & Bruno, J. P. (2001). The cognitive neuroscience of sustained attention: Where top-down meets bottom-up. Brain Research Reviews, 35(2), 146–160. https://doi.org/10.1016/S0165-0173(01)00044-3
Sattin, R. W., Lambert Huber, D. A., Devito, C. A., Rodriguez, J. G., Ros, A., Bacchelli, S., … Waxweiler, R. J. (1990). The incidence of fall injury events among the elderly in a defined population. American Journal of Epidemiology, 131(6), 1028–1037. https://doi.org/10.1093/oxfordjournals.aje.a115594
Schneider, W., Eschman, A., Zuccolotto, A., & Guide, E. (2002). Psychology Software Tools Inc. Pittsburgh, USA.
Schwartz, & Fargo, J. (2017). USU – PSY 7650.
Seth, A. K. (2010). A MATLAB toolbox for Granger causal connectivity analysis. Journal of Neuroscience Methods, 186(2), 262–273. https://doi.org/10.1016/j.jneumeth.2009.11.020
Seth, A. K., Barrett, A. B., & Barnett, L. (2015). Granger Causality Analysis in Neuroscience and Neuroimaging. Journal of Neuroscience, 35(8). Retrieved from http://www.jneurosci.org/content/35/8/3293.short
Shephard, R. J. (1988). PAR-Q, Canadian Home Fitness Test and Exercise Screening Alternatives. Sports Medicine, 5(3), 185–195. https://doi.org/10.2165/00007256-198805030-00005
Shumway-Cook, A., & Woollacott, M. H. (1995). Motor Control Theory And Practical Applications.
Shumway-Cook, A., & Woollacott, M. H. (2017). Motor control : translating research into clinical practice.
Shumway-Cook A, & Brauer S. (2000). Research report predicting the probability for falls in community-dwelling older adults using the timed up & go test. Phys Ther., 80(9), 896–903.
Simoes, M. A. (2011). Feasibility of Wearable Sensors to Determine Gait Parameters. Department of Mechanical Engineering, Master of, 108. https://doi.org/10.1007/s13398-014-0173-7.2
Smania, N., Corato, E., Tinazzi, M., Stanzani, C., Fiaschi, A., Girardi, P., & Gandolfi, M. (2010). Effect of Balance Training on Postural Instability in Patients With Idiopathic Parkinson’s Disease. Neurorehabilitation and Neural Repair, 24(9), 826–834. https://doi.org/10.1177/1545968310376057
Smith-Ray, R. L., Makowski-Woidan, B., & Hughes, S. L. (2014). A Randomized Trial to Measure the Impact of a Community-Based Cognitive Training Intervention on Balance and Gait in Cognitively Intact Black Older Adults. Health Education & Behavior, 41(1_suppl), 62S–69S. https://doi.org/10.1177/1090198114537068
Stelmach, G. E., Teasdale, N., Di Fabio, R. P., & Phillips, J. (1989). Age Related Decline in Postural Control Mechanisms. The International Journal of Aging and Human Development, 29(3), 205–223. https://doi.org/10.2190/KKP0-W3Q5-6RDN-RXYT
Stelmach, G. E., & Worringham, C. J. (1985). Sensorimotor deficits related to postural stability. Implications for falling in the elderly. Clinics in Geriatric Medicine, 1(3), 679–694. https://doi.org/10.1001/archsurg.140.1.54
Stevens, J. ., Corso, P. ., Finkelstein, E. ., & Miller, T. . (2006). The costs of fatal and non-fatal falls among older adults. Injury Prevention, 12, 290–295. Retrieved from http://injuryprevention.bmj.com/content/injuryprev/12/5/290.full.pdf
Teasdale, N., & Simoneau, M. (2001). Attentional demands for postural control: the effects of aging and sensory reintegration. Gait & Posture, 14(3), 203–210. https://doi.org/10.1016/S0966-6362(01)00134-5
Thomas, S., Reading, J., & Shephard, R. J. (1992). Revision of the Physical Activity Readiness Questionnaire (PAR-Q). Canadian Journal of Sport Sciences, 17(4), 338–345.
Tinetti, M. E., Speechley, M., & Ginter, S. F. (1988). Risk Factors for Falls among Elderly Persons Living in the Community. New England Journal of Medicine, 319(26), 1701–1707. https://doi.org/10.1056/NEJM198812293192604
Visser, J. E., Carpenter, M. G., van der Kooij, H., & Bloem, B. R. (2008). The clinical utility of posturography. Clinical Neurophysiology, 119(11), 2424–2436. https://doi.org/10.1016/j.clinph.2008.07.220
Washabaugh, E. P., Kalyanaraman, T., Adamczyk, P. G., Claflin, E. S., & Krishnan, C. (2017). Validity and repeatability of inertial measurement units for measuring gait parameters. Gait and Posture, 55(October 2016), 87–93. https://doi.org/10.1016/j.gaitpost.2017.04.013
Woollacott, M. H. (2000). Editorial: Systems Contributing to Balance Disorders in Older Adults. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 55(8), M424–M428. https://doi.org/10.1093/gerona/55.8.M424
Woollacott, M. H., Shumway-Cook, A., & Nashner, L. M. (1986). Aging and Posture Control: Changes in Sensory Organization and Muscular Coordination. The International Journal of Aging and Human Development, 23(2), 97–114. https://doi.org/10.2190/VXN3-N3RT-54JB-X16X
Woollacott, M., & Shumway-Cook, A. (2002a). Attention and the control of posture and gait: a review of an emerging area of research. Gait & Posture, 16(1), 1–14. https://doi.org/10.1016/S0966-6362(01)00156-4
Woollacott, M., & Shumway-Cook, A. (2002b). Attention and the control of posture and gait: A review of an emerging area of research. Gait and Posture, 16(1), 1–14. https://doi.org/10.1016/S0966-6362(01)00156-4
Xu, J., Liu, X., Zhang, J., Li, Z., Wang, X., Fang, F., & Niu, H. (2015). FC-NIRS: A Functional Connectivity Analysis Tool for Near-Infrared Spectroscopy Data. BioMed Research International, 2015. https://doi.org/10.1155/2015/248724
Yardley, L. (2001). Interference between postural control and mental task performance in patients with vestibular disorder and healthy controls. Journal of Neurology, Neurosurgery & Psychiatry, 71(1), 48–52. https://doi.org/10.1136/jnnp.71.1.48
Ye, J. C., Tak, S., Jang, K. E., Jung, J., & Jang, J. (2009a). NIRS-SPM: Statistical parametric mapping for near-infrared spectroscopy. NeuroImage, 44(2), 428–447. https://doi.org/10.1016/j.neuroimage.2008.08.036
Ye, J. C., Tak, S., Jang, K. E., Jung, J., & Jang, J. (2009b). NIRS-SPM: Statistical parametric mapping for near-infrared spectroscopy. NeuroImage, 44(2), 428–447. https://doi.org/10.1016/j.neuroimage.2008.08.036
Appendix

: IRB Consent form


Medical History Questionnaire
Cognition- balance study
Medical History Questionnaire
Group: Young/ Old
This is your medical history form, to be completed prior to your first training session. All information will be kept confidential. This information will be used for the evaluation of your health and readiness to begin our exercise program. Please take your time and complete it carefully and thoroughly, and then review it to be certain you have not left anything out. Your answers will help us design a comprehensive program that meets your individual needs.
If you have questions or concerns, we will help you with those after this form is completed. We realize that some parts of the form will be unclear to you. Do your best to complete the form. Your questions will be thoroughly addressed afterwards.
| Participant #: | Date of study: |
| Height: | Weight: |
| Date of birth: | Contact info |
Sex:
Male Female
Education:
Grade School Jr. High School High School
College (2-4 years) Graduate School Degree _______________
Do you have difficulties in getting up from a seated position? Yes No
Do you have any hearing difficulties? Yes No
Comments: __________________________________________________________________
Do you have any lower-extremity injury/ies? Yes No
If yes,
When did you have the injury? _________________________________________________
How many injuries have you had? _______________________________________________
Comments: __________________________________________________________________
Have you had any concussions in the past 12 weeks? Yes No
If yes,
When did you have the last concussion? ________________________________________
How many concussions did you have? __________________________________________
Comments: __________________________________________________________________
In a week do you perform any physical activity/ies? Yes No
If yes,
What are the activities? ________________________________________________________
How many times per week do you perform these activities?
___________________________________________________________________________
Men and women answer the following:
List any prescription medications you are now taking:
List any self-prescribed medications, dietary supplements, or vitamins you are now taking:
Past Medical History
Check those questions to which your answer is yes (leave others blank).
- Arthritis of legs or arms
- Epilepsy or seizures
- Stroke
 Montreal Cognitive Assessment (MoCA)
Montreal Cognitive Assessment (MoCA)
Edinburgh Handedness form

Physical Activity Readiness Questionnaire (PAR-Q)

TOMAL-2: Letters Forward Subtest
Participant data sheet
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Physiology"
Physiology is related to biology, and is the study of living organisms and how they function. Physiology covers all living organisms, exploring how the body performs basic functions in relation to physics and chemistry.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




