Ocular Disease Severity Varies by Pathogen in Cats
Info: 3573 words (14 pages) Dissertation
Published: 9th Dec 2019
Tagged: Animal Sciences
Ocular disease severity varies by pathogen in cats.
Keywords: feline, PCR, feline herpesvirus, feline calicivirus, Chlamydophila felis, feline coronavirus, Mycoplasma felis, ocular
Objectives
Feline herpesvirus-1 (FHV-1) has previously been implicated in causing feline ocular disease. The objective of this study was to identify pathogens using PCR and viral isolation in shelter cats with ocular and upper respiratory disease, and to identify factors associated with increased severity of clinical disease.
Methods
Oropharyngeal swabs from 90 cats in 11 animal shelters across the USA were obtained and tested by real-time PCR using a feline respiratory panel after clinical scoring for ocular and respiratory disease. A multivariable linear mixed model assessed the relationship between clinical scores and age, sex and pathogen. Viral isolation was performed on all samples, then 42/90 underwent DNA extraction and gel electrophoresis.
Results
Five pathogens were detected by real-time PCR: feline calicivirus (FCV) (63%), FHV-1 (54%), Mycoplasma felis (50%), feline coronavirus (FCoV) (17%) and Chlamydophila felis (3%). A positive FHV-1 PCR result was associated with slightly higher respiratory clinical scores (95% CI= (0.050-1.351), p <0.05) and lower ocular clinical scores (95%CI= (-2.464,-0.597), p <0.01). Male cats (n=51) had higher ocular clinical scores than females (95%CI= (0.342-1.789), p < 0.01). Viral isolation detected virus in 84/90 samples within 7 days. Virus isolates from 18/21 samples with both FHV-1 and FCV detected by PCR showed no banding consistent with FHV-1 on electrophoresis after DNA extraction. Of these 18 samples, 15 were confirmed to contain FCV by PCR performed on first viral passage supernatant.
Conclusions and Relevance
Our finding that FHV-1 was associated with higher respiratory scores, but lower ocular scores, contradicts current understanding and warrants further research. The results of the viral isolation demonstrate that FCV may grow preferentially over FHV-1 in vitro. Overall, the results indicate that FCV may play a more significant role in infectious ocular disease in cats housed in animal shelters than previously realized.
Introduction
Upper respiratory disease, both with and without ocular involvement, is an extremely common problem in shelter cats and the companion animal population (1). Common pathogens suggested to be responsible in the past include feline herpesvirus-1 (FHV-1), feline calicivirus (FCV), Chlamydophila felis, feline coronavirus (FCoV) and Mycoplasma felis. Specifically, FHV-1 has been associated with respiratory disease (2-4), ocular disease (2, 4) and skin disease (5). FCV has been associated with gingivostomatitis (6-12), respiratory disease (2, 3, 11) and ocular disease (2, 11). C.felis has been associated with respiratory disease (2) and ocular disease (2, 13). FCoV has been reported to cause mild respiratory signs, diarrhea and can mutate to cause feline infectious peritonitis (14). M. felis has been identified as a cause of pneumonia in cats (15), upper respiratory disease (16) and has been associated with ocular disease (13, 17-20). Current recommendations are that cats in a shelter environment should be vaccinated against both FHV-1 and FCV (21-23).
Previous studies have looked at the prevalence of these pathogens through the use of PCR in Spain (2), Switzerland (8), UK (24, 25), Germany (11), Canada (26, 27), Europe (3), Brazil (28), Korea (29), Japan (30), Australia (31) and the USA (32-34). However, the previous published studies that have taken place in the USA have been restricted geographically to California (32), New England (33) and Colorado (34).
Both ocular and oropharyngeal swabs from cats have been used to perform PCR for upper respiratory disease pathogens. The use of oropharyngeal swabs may increase the detection rate of pathogens (35).
The primary objectives of this study were to perform a survey of the pathogens causing upper respiratory and ocular disease in shelter cats across the USA, to identify associated pathogens using PCR and viral isolation, and to identify factors associated with increased severity of clinical disease in affected animals.
Materials and methods
Sample Acquisition and PCR
All procedures were performed in accordance with an approved University of Wisconsin-Madison Institutional Animal Care and Use Committee protocol. Cats in shelters with signs of respiratory disease with or without ocular involvement were identified by shelter veterinarians in eleven geographically distinct areas of the USA (Table 1, Figure 1). The veterinarians were instructed to include any animals showing clinical signs of respiratory disease of any severity with or without ocular involvement. The number of samples submitted from each location was limited by the number of animals showing clinical signs at the time of sampling. Samples were submitted between the months of August and December. Veterinarians at each shelter used a clinical scoring system (Table 2) to create a clinical score for each cat. The clinical signs were designated as either ocular, respiratory or generalized (Table 2). Two oropharyngeal swabs were taken from each cat by brushing the oropharyngeal area firmly for around 30 seconds. The swabs were then placed into a transport medium (Universal Viral Transport, Becton, Dickinson and Company), labelled and double bagged to prevent cross-contamination. Gloves were changed between animals. The swabs were shipped overnight to the Wisconsin Veterinary Diagnostic Laboratory for feline respiratory panel testing (FHV-1, FCV, C.felis, FCoV and M. felis) by real-time PCR and to the UW-Madison Brandt laboratory for viral isolation. The PCR test was validated as per accreditation protocols set by the American Association of Veterinary Laboratory Diagnosticians.
Initial viral isolation and creation of viral stocks
Samples were immediately refrigerated on receipt prior to viral isolation. A 1 ml aliquot of each sample was added to individual 100 mm tissue culture plates with maximally confluent Crandell Rees feline kidney cells (CRFK) along with 1 ml of Dulbecco’s modified Eagle’s medium containing 2% fetal bovine serum, 100 units/ml penicillin, 100 µg/ml streptomycin sulfate and 250 µg/ml amphotericin B (DMEM) before being incubated at 37°C for 60 minutes. An additional 4 ml of DMEM was then added to each plate before being incubated at 37°C and checked daily for 7 days until 100% cytopathic effect (CPE) was observed. The cells and media were scraped and pipetted from the plate and placed in a conical tube for centrifugation at 600 X g for 10 minutes at 4°C in a Sorvall X1R Legend centrifuge. The supernatant was removed and the pellet was re-suspended in 750 µL of the reserved culture medium. The remainder of the culture supernatant was stored at 4°C. The re-suspended pellet was subjected to three freeze-thaw cycles and centrifuged at 600 X g for 10 minutes at 4°C (Sorvall X1R Legend centrifuge). The resultant supernatant was combined with the saved culture medium and stored in 200 µL aliquots at -80°C.
Viral DNA preparation
Viral DNA was prepared using a modification of a previously published protocol (36). Briefly, a thawed vial of virus stock was added to 12ml of DMEM in a 15 ml conical tube. Two ml of this mixture per plate was added to 6 confluent 100 mm tissue culture plates of CRFK cells and incubated at 37°C for 60 minutes. An additional 4 ml of DMEM was added to each plate before being incubated at 37°C and checked daily until 100% CPE was observed. The cells and media were scraped and added to a single 50 ml conical tube before being centrifuged at 600 X g for 10 minutes at 4°C (Sorvall Legend X1R centrifuge). The supernatant was then stored at 4°C. The pellets were re-suspended in 5 ml of the saved supernatant and subjected to three freeze-thaw cycles. All supernatants were combined and centrifuged at 600 X g for 10 minutes at 4°C. The resultant supernatant was then centrifuged at 600 X g for 5 minutes at 4°C. The supernatant was layered onto a 36% sucrose cushion in 0.1 M phosphate-buffered saline and centrifuged for 80 min at 24,000 X g at 4°C (Sorvall WX Ultra Series ultracentrifuge). The pellet was re-suspended in 1 ml of TE buffer per tube (10 mM Tris [pH 7.4], 1 mM EDTA) with 0.15 M sodium acetate and 50 μg/ml RNase A and then incubated for 15 min at 37°C. Proteinase K and SDS (50 μg/ml and 0.1%, final concentrations respectively) were added, and the solution was incubated for 15 min at 37°C. The viral DNA was purified by phenol-chloroform extraction and ethanol precipitation, incubated with 50 μg/ml RNase A for a further 15 minutes, re-suspended in deionized water, and stored at −20°C. DNA purity and concentration was analyzed using a Nanodrop Lite Spectrophotometer (Thermo Scientific).
Gel Electrophoresis and PCR Confirmation of Pathogens
Viral DNA samples obtained from second viral passage supernatants were digested with Bam H1 according to the manufacturer’s instructions (Promega) for 12 hours at 37°C and electrophoresed in 0.8% agarose. DNA from a plaque-purified field isolate that was verified as FHV-1 by immunofluorescence with FHV–1-specific antiserum (37) was included as a positive control.
Supernatant obtained after first passage of each virus which was positive for both FHV-1 and FCV on PCR but showed no banding consistent with FHV-1 on electrophoresis after DNA extraction was submitted to the Wisconsin Veterinary Diagnostic Laboratory for real-time PCR panel testing (FHV-1, FCV).
Statistics
The following variables were analyzed statistically; total clinical score, respiratory clinical score, ocular clinical score, generalized signs clinical score, sex (male/female), age (juvenile, ≤ 5 months; young, 6-23 months; adult, ≥ 24 months), PCR status for FHV-1/FCV/FCoV/M. felis/C. felis (positive or negative) and geographic collection site (Eastern USA, Western USA, Southern USA, Midwestern USA). For the purposes of clustering the shelters by geographic location, designations as shown in Table 1 were used. Before fitting a multivariable model to the data, different univariate relationships in the data were explored. The effect of geographic collection site, sex, age, total number of pathogens per sample, PCR status for pathogens and prior anti-viral use on different clinical scores were explored graphically (38) and numerically when appropriate through the use of non-parametric testing procedures (Kruskal-Wallis or Mann-Whitney rank sum tests).
Following the initial exploration of the data it was decided that the relationship between both FHV-1 and FCV and ocular and respiratory clinical scores would be the primary focus of further investigation.
To estimate the relationship between FHV-1 and both ocular and respiratory clinical scores, two separate linear mixed models (LMM) were fit to the data (one for each clinical symptom score) using the restricted maximum likelihood (REML) criterion in the lme4 (V 1.1-12) package for R (V 3.3.2) (39, 40). Both models were adjusted for age, sex, total number of pathogens detected by PCR, FCV PCR status, FCoV PCR status and FHV-1 PCR status, while collection site was modeled as a random effect. For the respiratory model, the random effect variance was estimated to be zero by the model, hence the LMM estimates were identical to those of a fixed-effects multiple linear regression. A more complex model, which included a random effect for the region of the shelter (with an identical fixed-effects specification) was found to not fit the data significantly better than our proposed model (LRT
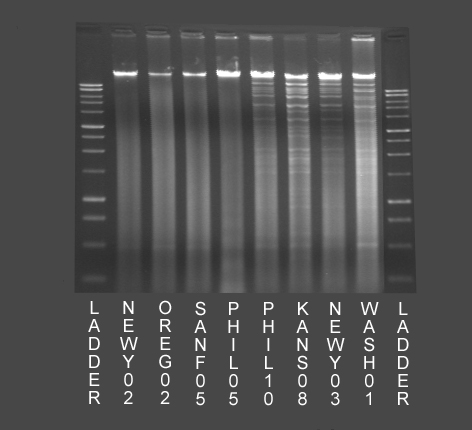
Figure 3 – Examples of results of DNA extraction and gel electrophoresis of viral isolates from 8 cats. The 4 lanes on the left of the image (NEWY02, OREG02, SANF05 and PHIL05, all FHV-1 PCR positive and FCV PCR positive) do not demonstrate banding typical of the presence of Feline Herpesvirus-1 DNA. The 4 lanes on the right of the image (PHIL10, KANS08, NEWY03 and WASH01, all FHV-1 PCR positive and FCV PCR negative) demonstrate banding typical of the presence of Feline Herpesvirus-1 DNA
References
- Cohn LA. Feline respiratory disease complex. Vet Clin North Am Small Anim Pract. 2011;41(6):1273-89.
- Fernandez M, Manzanilla EG, Lloret A, León M, Thibault J-C. Prevalence of feline herpesvirus-1, feline calicivirus, Chlamydophila felis and Mycoplasma felis DNA and associated risk factors in cats in Spain with upper respiratory tract disease, conjunctivitis and/or gingivostomatitis. J Feline Med Surg. 2016:1098612X16634387.
- Helps CR, Lait P, Damhuis A, Björnehammar U, Bolta D, Brovida C, et al. Factors associated with upper respiratory tract disease caused by feline herpesvirus, feline calicivirus, Chlamydophila felis and Bordetella bronchiseptica in cats: experience from 218 European catteries. Vet Rec. 2005;156(21):669-73.
- Gaskell R, Dawson S, Radford A, Thiry E. Feline herpesvirus. Veterinary research. 2007;38(2):337-54.
- Johnson RP, Sabine M. The isolation of herpesviruses from skin ulcers in domestic cats. Vet Rec. 1971;89(13):360-2.
- Addie DD, Radford A, Yam PS, Taylor DJ. Cessation of feline calicivirus shedding coincident with resolution of chronic gingivostomatitis in a cat. J Small Anim Pract. 2003;44(4):172-6.
- Belgard S, Truyen U, Thibault JC, Sauter-Louis C, Hartmann K. Relevance of feline calicivirus, feline immunodeficiency virus, feline leukemia virus, feline herpesvirus and Bartonella henselae in cats with chronic gingivostomatitis. Berl Munch Tierarztl Wochenschr. 2010;123(9-10):369-76.
- Berger A, Willi B, Meli ML, Boretti FS, Hartnack S, Dreyfus A, et al. Feline calicivirus and other respiratory pathogens in cats with Feline calicivirus-related symptoms and in clinically healthy cats in Switzerland. BMC Vet Res. 2015;11(1):282.
- Dolieslager SM, Lappin DF, Bennett D, Graham L, Johnston N, Riggio MP. The influence of oral bacteria on tissue levels of Toll-like receptor and cytokine mRNAs in feline chronic gingivostomatitis and oral health. Vet Immunol Immunopathol. 2013;151(3-4):263-74.
- Dowers KL, Hawley JR, Brewer MM, Morris AK, Radecki SV, Lappin MR. Association of Bartonella species, feline calicivirus, and feline herpesvirus 1 infection with gingivostomatitis in cats. J Feline Med Surg. 2010;12(4):314-21.
- Gerriets W, Joy N, Huebner-Guthardt J, Eule JC. Feline calicivirus: a neglected cause of feline ocular surface infections? Vet Ophthalmol. 2012;15(3):172-9.
- Lommer MJ, Verstraete FJ. Concurrent oral shedding of feline calicivirus and feline herpesvirus 1 in cats with chronic gingivostomatitis. Oral Microbiol Immunol. 2003;18(2):131-4.
- Hartmann AD, Hawley J, Werckenthin C, Lappin MR, Hartmann K. Detection of bacterial and viral organisms from the conjunctiva of cats with conjunctivitis and upper respiratory tract disease. J Feline Med Surg. 2010;12(10):775-82.
- Giori L, Giordano A, Giudice C, Grieco V, Paltrinieri S. Performances of different diagnostic tests for feline infectious peritonitis in challenging clinical cases. J Small Anim Pract. 2011;52(3):152-7.
- Chandler JC, Lappin MR. Mycoplasmal respiratory infections in small animals: 17 cases (1988–1999). J Am Anim Hosp Assoc. 2002;38(2):111-9.
- Lee-Fowler T. Feline respiratory disease: what is the role of Mycoplasma species? J Feline Med Surg. 2014;16(7):563-71.
- Tan RJ. Suceptibility of kittens to Mycoplasma felis infection. Jpn J Exp Med. 1974;44(3):235-40.
- Campbell LH, Snyder SB, Reed C, Fox JG. Mycoplasma felis-associated conjunctivitis in cats. J Am Vet Med Assoc. 1973;163(8):991-5.
- Haesebrouck F, Devriese LA, van Rijssen B, Cox E. Incidence and significance of isolation of Mycoplasma felis from conjunctival swabs of cats. Vet Microbiol. 1991;26(1-2):95-101.
- Low HC, Powell CC, Veir JK, Hawley JR, Lappin MR. Prevalence of feline herpesvirus 1, Chlamydophila felis, and Mycoplasma spp DNA in conjunctival cells collected from cats with and without conjunctivitis. Am J Vet Res. 2007;68(6):643-8.
- Möstl K, Egberink H, Addie D, Frymus T, Boucraut-Baralon C, Truyen U, et al. Prevention of infectious diseases in cat shelters: ABCD guidelines. J Feline Med Surg. 2013;15(7):546-54.
- Radford AD, Addie D, Belák S, Boucraut-Baralon C, Egberink H, Frymus T, et al. Feline calicivirus infection. ABCD guidelines on prevention and management. J Feline Med Surg. 2009;11(7):556-64.
- Thiry E, Addie D, Belák S, Boucraut-Baralon C, Egberink H, Frymus T, et al. Feline herpesvirus infection. ABCD guidelines on prevention and management. J Feline Med Surg. 2009;11(7):547-55.
- Binns SH, Dawson S, Speakman AJ, Cuevas LE, Hart CA, Gaskell CJ, et al. A study of feline upper respiratory tract disease with reference to prevalence and risk factors for infection with feline calicivirus and feline herpesvirus. J Feline Med Surg. 2000;2(3):123-33.
- Edwards DS, Coyne K, Dawson S, Gaskell RM, Henley WE, Rogers K, et al. Risk factors for time to diagnosis of feline upper respiratory tract disease in UK animal adoption shelters. Prev Vet Med. 2008;87(3-4):327-39.
- Gourkow N, Lawson JH, Hamon SC, Phillips CJ. Descriptive epidemiology of upper respiratory disease and associated risk factors in cats in an animal shelter in coastal western Canada. Can Vet J. 2013;54(2):132-8.
- Sandmeyer LS, Waldner CL, Bauer BS, Wen X, Bienzle D. Comparison of polymerase chain reaction tests for diagnosis of feline herpesvirus, Chlamydophila felis, and Mycoplasma spp. infection in cats with ocular disease in Canada. Can Vet J. 2010;51(6):629-33.
- Henzel A, Brum MC, Lautert C, Martins M, Lovato LT, Weiblen R. Isolation and identification of feline calicivirus and feline herpesvirus in Southern Brazil. Braz J Microbiol. 2012;43(2):560-8.
- Kang BT, Park HM. Prevalence of feline herpesvirus 1, feline calicivirus and Chlamydophila felis in clinically normal cats at a Korean animal shelter. J Vet Sci. 2008;9(2):207-9.
- Mochizuki M, Kawakami K, Hashimoto M, Ishida T. Recent epidemiological status of feline upper respiratory infections in Japan. J Vet Med Sci. 2000;62(7):801-3.
- Wong W, Kelman M, Ward M. Surveillance of upper respiratory tract disease in owned cats in Australia, 2009–2012. Prev Vet Med. 2013;112(1):150-5.
- Bannasch MJ, Foley JE. Epidemiologic evaluation of multiple respiratory pathogens in cats in animal shelters. J Feline Med Surg. 2005;7(2):109-19.
- Dinnage JD, Scarlett JM, Richards JR. Descriptive epidemiology of feline upper respiratory tract disease in an animal shelter. J Feline Med Surg. 2009;11(10):816-25.
- Veir JK, Ruch-Gallie R, Spindel ME, Lappin MR. Prevalence of selected infectious organisms and comparison of two anatomic sampling sites in shelter cats with upper respiratory tract disease. J Feline Med Surg. 2008;10(6):551-7.
- Schulz C, Hartmann K, Mueller RS, Helps C, Schulz BS. Sampling sites for detection of feline herpesvirus-1, feline calicivirus and Chlamydia felis in cats with feline upper respiratory tract disease. J Feline Med Surg. 2015;17(12):1012-9.
- Kintner RL, Brandt CR. Rapid small-scale isolation of herpes simplex virus DNA. J Virol Methods. 1994;48(2-3):189-96.
- Nasisse MP, Guy JS, Davidson MG, Sussman WA, Fairley NM. Experimental ocular herpesvirus infection in the cat. Sites of virus replication, clinical features and effects of corticosteroid administration. Invest Ophthalmol Vis Sci. 1989;30(8):1758-68.
- Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag; 2009.
- Bates D, Maechler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw. 2015;67(1):1-48.
- RCoreTeam. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2016.
- Hartley C. Aetiology of corneal ulcers assume FHV-1 unless proven otherwise. J Feline Med Surg. 2010;12(1):24-35.
- Gould D. Feline herpesvirus-1: ocular manifestations, diagnosis and treatment options. J Feline Med Surg. 2011;13(5):333-46.
- Quimby JM, Elston T, Hawley J, Brewer M, Miller A, Lappin MR. Evaluation of the association of Bartonella species, feline herpesvirus 1, feline calicivirus, feline leukemia virus and feline immunodeficiency virus with chronic feline gingivostomatitis. J Feline Med Surg. 2008;10(1):66-72.
- Maggs DJ, Lappin MR, Reif JS, Collins JK, Carman J, Dawson DA, et al. Evaluation of serologic and viral detection methods for diagnosing feline herpesvirus-1 infection in cats with acute respiratory tract or chronic ocular disease. J Am Vet Med Assoc. 1999;214(4):502-7.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Animal Sciences"
Animal science can be described as studying the biochemistry, physiology, and behaviour of animals that are under human control. Historically, animal science degrees were known as animal husbandry and focused on livestock. Studies now include companion animals such as cats and dogs.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




