Neutralisation of Stomach Acid by Magnesium Hydroxide Antacid
Info: 11049 words (44 pages) Dissertation
Published: 17th Aug 2021
Tagged: MedicalPharmacology
Abstract
An experimental investigation was conducted to examine the chemical concepts of acid-base chemistry and equilibria in the form of a neutralisation reaction, with real world applications of indigestion in the human body. Using an antacid with the active ingredient of magnesium hydroxide (Mg(OH)2), it was hypothesised that as the mass of antacid was increased, the hydrochloric acid will require less sodium hydroxide to neutralise through a back titration, supported by quantitative evidence of pH testing. Following preliminary data collection, modifications were applied to the method and materials required for the experiment through the completion of three experimental trials. The results attained from the experiments partially supported the hypothesis, because as the amount of antacid added increased, there was a negative slope in the amount of NaOH needed to neutralise the base, until the dose of two tablets, which refuted the hypothesis as it needed more NaOH than the previous dose of 1 ½ tablets. Additionally, the pH data attained supported the hypothesis in the manner that the results showed that the more antacid was added, the more the solution was neutralised. Theories of chemistry including Le Chatelier and the Bronsted-Lowry Concepts theoretically support the findings of the experiment, which correlate to current scientific knowledge on the human body and indigestion.
Contents
4.0 Preliminary Investigations
8.0 Analysis of Results and Discussion
9.0 Recommendations and Conclusion
12.1 Appendix 1 – Calculations
1.0 Introduction
Indigestion, otherwise known as dyspepsia or acid reflux, is a biologically common issue within humans and numerous other organisms. Resulting from an increase in the secretion of hydrochloric acid within the stomach, of which can have both long and short-term repercussions, one of the quickest and easiest ways to neutralise the acid and stop the painful burn is to consume an antacid tablet. In order to investigate this concept of indigestion and the use of antacids to neutralise stomach acid, of which is the primary purpose of the experimental analysis, the chemical principles of neutralisation and equilibrium in acid-base reactions can be applied. Focusing on the biochemical process, the investigation will consider the intrinsic association between neutralisation and the consequential changes within pH levels, with the subsequent application of said chemical principles.
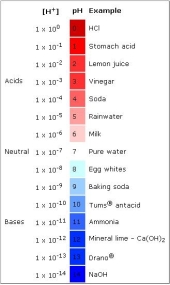
The pH scale, or ‘Power of Hydrogen’ scale, is a logarithmic measurement of the molar concentration of hydrogen ions in a solution, which subsequently is a measure of how acidic or basic (alkaline) a substance is (Ophardt, 2003). Measured on a scale from 0-14, zero being the most acidic and fourteen being the most basic, the numerical value can be calculated through using the equation below and is visually represented on the scale to the right (Clark, 2013).
Equation 1:
pH= -log10[H+]
The negative base 10 logarithm equation is vital in the comprehension and understanding of foundational concepts within chemistry. In particular and in reference to this experiment, the Bronsted-Lowry and Arrhenius theories of acids and bases can be used to explain the chemical process behind neutralisation of stomach acid.
Figure 1: The pH scale and examples of corresponding pH of everyday items. Source: (Smithsakol, 2018).
As special types of electrolytes, acids and bases are known for breaking up into the charged particles of hydron ions (H+) and hydroxide ions (OH–). The theory of Arrhenius acids and bases explains that an acid is any species of which is capable of increasing its H+ concentration in an aqueous solution and which ionise and produce a hydronium ion (H3O+), whereas a base is any species of which increases the concentration of OH– ions in an aqueous solution (Khan Academy, 2018). However, while the concept can be applied to chemical reactions between acids and bases, it only complies with aqueous environments. Conversely, the Bronsted-Lowry, while not replacing the Arrhenius theory but using it as a foundation, describes interactions between acids and bases as one of proton transfer amid chemical species (Lumen Learning, 2018). Instead, an acid can be described as a proton donor (H+), compared to a base, which is a proton acceptor. This concept of acid and base theory is more widely recognised and accepted in chemistry due to the broad range of reactions and chemicals the theory can be applied to.
Within acids and bases, varying types of each within a reaction will determine whether an acid or base is strong or weak. For example, strong acids and bases refer to species that wholly dissociate in order to form ions within a solution; as compared to weak acids and bases which partially ionise, in which the reaction is reversible (Khan Academy, 2018). The strength of an acid is dependent on the amount it dissociates, whereby acids increase in strength the more they dissociate. This can be quantified by the acid dissociation constant, known as Ka and can be numerically quantified by the equation below for the example of hydrochloric acid, where the square brackets represent the concentration of the substance inside (Wyman, 2018):
Equation 2:
Ka=H+[Cl-][HCl]
The equation is a ratio of products to reactants, whereby the more HCl dissociates into H+ and the conjugate base Cl–, the stronger the acid and thus the closer the value is to one. As a strong base, hydrochloric acid fully dissociates, and thus on a molecular level, no HCl molecules can be found because they dissociate into hydrogen and chlorine ions. Additionally, the hydrogen ions have attached to oxygen molecules, subsequently forming hydronium ions (H3O+). The dissociation of hydrochloric acid is represented in the equation below (Ryan, 2018):
Equation 3:
HClg+H2Ol←→H3Oaq+Cl-(aq)
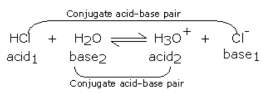 This reaction can be explained through the Bronsted-Lowry concept, whereby the HCl is an acid as it is donating a proton (the hydrogen ion) to the H2O, which is a base because it is accepting the proton, thus making it a donor (Khan Academy, 2018). However, the reaction is reversible, as the H3Ois capable of donating a hydrogen ion to the Cl– ion, thus making it an acid, and this ion is a base because it is accepting the proton. As the reaction contains two pairs of acids and bases, these pairs are called conjugate pairs and can be seen below.
This reaction can be explained through the Bronsted-Lowry concept, whereby the HCl is an acid as it is donating a proton (the hydrogen ion) to the H2O, which is a base because it is accepting the proton, thus making it a donor (Khan Academy, 2018). However, the reaction is reversible, as the H3Ois capable of donating a hydrogen ion to the Cl– ion, thus making it an acid, and this ion is a base because it is accepting the proton. As the reaction contains two pairs of acids and bases, these pairs are called conjugate pairs and can be seen below.
Figure 2: Hydrochloric acid as an example of a conjugate pair. Source: (Ellesmere OCR, 2015)
In reacting acids and bases, a neutralisation reaction can occur, where an acid and base react to form the product of salt and water. The process combines H+ ions from the acid and OH– ions from the base to generate water and thus lessens the acidic and basic properties of both solutions (Chemistry LibreTexts, 2018). When a strong acid and base completely neutralise, for example, hydrochloric acid and sodium hydroxide, the pH is neutral, or equal to seven. In this process, there are equivalent amounts of OH–and H3O+within the solution, with no surplus of NaOH (Chemistry LibreTexts, 2018).
Equation 4:
HClaq+NaOHaq←→NaClaq+H2O(l)
Another chemical principle, equilibrium, is a vital part of acid-base reactions. Chemical equilibrium is the state by which the forward and reverse reaction occur at equal rates, whereby the products and reactants remain constant (Rice University, 2015). Conversely, in dynamic equilibrium, products are converted into reactants and reactants are converted into products at a constant and equalised rate, however, most commonly does not result in equal concentrations (FuseSchool, 2013). Further explained through Le Chatelier’s Principle, whereby it is proposed “a change in one of the variables that describe a system at equilibrium produces a shift in the position of the equilibrium that counteracts the effects of this change” (Purdue University, 2018). Laws of equilibria define the direction that proceeds a chemical reaction, in addition to the quantities and concentrations remaining once the reaction concludes. Changing conditions of a reaction such as the concentration of a component, the pressure of the system, or changing the temperature at which the reaction is run can reverse the reaction or shift the reaction’s quantities until a new state of equilibrium is achieved (Lower, 2017). The reason for this alteration is the kinetic inhibition of the reaction, whereby an increase in one factor of a reaction will change the kinetic motion of particles within the reaction, either slowing or increasing the rate of a reaction. In regards to effects of antacid tablets on indigestion, and according to the principle, adding additional reactant to a system, or reducing the concentration will shift the equilibrium to the right. This reactant would be, in this regard, the antacid. Likewise, the reverse is true: the addition of excess products (the stomach acid) shifts the equilibrium to the left, until a new state of equilibrium is acquired. These reactions can be named ‘self-correcting’, as they naturally shift to restore a balance after change (Lumen Learning, 2018). Thus can be deduced that concepts of acid-base chemistry are pivotal to the explanation of neutralisation of stomach contents through use of antacids.
Through natural secretion of hydrochloric acid into the contents of the stomach, proteins are broken down through the acidic conditions created by said secretion, as the enzyme of pepsin is most active in this acidic environment (Ogbru, 2018). The lower oesophageal sphincter, a circular muscle separating the stomach and the oesophagus, is responsible for closing the entrance to the stomach upon the entrance of food. However, if it does not close or opens whilst food is still present in the stomach, due to the churning movement of physical digestion, a mixture of food and stomach acid may be pushed through the sphincter and into the lower portion of the oesophagus. This disrupts the usual pH of 7 within oesophagus, irritating its walls and creating a burning sensation known as heartburn or indigestion (Crampton, 2017).
Antacids, as one of the oldest and most effective remedies for indigestion and other stomach acid-related issues, are most commonly weak bases of which are used to affect and influence excess stomach acid, and its unusually low pH, through the chemical process of neutralisation (Eddy, 2016). However, while it is assumed that the antacid as a whole actively contributes to the counteraction of the acid, the active ingredient performs the main role of neutralisation. The most common active ingredients come in the form of carbonates, bicarbonates and hydroxides. For instance, the majority of companies use sodium bicarbonate (NaHCO3), magnesium hydroxide (Mg(OH)2), aluminium hydroxide (Al(OH)3), or calcium carbonate (CaCO3) as the weak base of which counteracts the stomach acid (International Foundation for Functional Gastrointestinal Disorders, 2015). This discussion will concentrate on the active ingredient of magnesium hydroxide (Mg(OH)2), of which is found in Mylanta® products (Johnson & Johnson Pacific Pty Limited, 2014).
Through the characteristics of its quick dissolution and rapid buffering effects, in addition to its rapid gastric acid neutralisation qualities and high acid neutralisation capacity, magnesium hydroxide (Mg(OH)2), otherwise known as milk of magnesia, is one of the most effective and widely used active ingredients for antacids (Ogbru, 2018). With low solubility in water and the quality of interfering with absorption of folic acid and iron, of which prevents and relieves varying causes of nausea, magnesium hydroxide is considered a strong electrolyte. Simultaneously, the substance’s Solubility Product Constant, Ksp, is of 1.5×10-11, meaning the amount of magnesium hydroxide that dissolves, also dissociates into its ions of Mg+ and OH– (Massachusetts Institute of Technology, 2010). Ksp can be defined as the equilibrium constant for a substance of solid disposition dissolving in an aqueous solution; a representative of the degree that a compound dissociates (Rashe & Peterson, 2016). Due to this limited solubility in water, magnesium hydroxide is considered an ideal compound because rather than dissolving wholly at once, it slowly dissolves as it neutralises. An indication of the compound’s low solubility can also be rendered from the equilibrium reaction that is far to the left, showing only a small amount of dissociation (Massachusetts Institute of Technology, 2010),
Equation 5:
Mg(OH)2s←→Mg2+aq+2OH-(aq)
As an antacid, generally dosed from 1-2 tablets by consumption, the reaction is one of neutralisation, where hydroxide ions from the compound bond to H+ions from the hydrochloric acid, which in turn, produce water, in accordance to the below equation
Equation 6:
H+aq+OH-aq→H2O(l)
The formation of water subsequently expends some of the OH–ions, shifting equation 5 to the right and consequenting in the dissolution of more magnesium hydroxide into its dissociative ions (Flinn Scientific, 2016). In adherence to Le Chatelier’s Principle, the more hydroxide ions that are removed from the solution due to their bonding with H+ions forces more magnesium hydroxide to dissociate in order to replace these ions. The reaction continues until the entirety of the antacid has been dissolved, leaving equation 5 lying fully to the right, meaning the acid-neutralising ability of the antacid has been exhausted (Flinn Scientific, 2016).
As indigestion is a biologically prominent and common issue within human digestion processes, a comprehensive understanding of the chemical concepts within the procedure is imperative in explaining the real world solution to indigestion: antacids in the form of magnesium hydroxide. The experimental investigation explores through back titration processes that magnesium hydroxide antacid tablets are capable of neutralising hydrochloric acid.
1.1 Aim
The aim of this experiment is to investigate the concept of indigestion, in conjunction with the chemical principles of neutralisation and equilibrium of acid-base reactions using quantitative analysis.
1.2 Hypothesis
It was hypothesised that as the amount (measured in grams) of antacid with the active ingredient of magnesium hydroxide (Mg(OH)2) increases from approximately 1.1g to 4.5g (
12a tablet to 2 tablets), the hydrochloric acid (HCl) will be neutralised with less sodium hydroxide in a back titration. Thus, pushing the equilibrium reaction to the right, moving the pH of the solution closer to seven. This prediction is based on the principles of neutralisation and equilibrium of acids and bases, as well as the biological process of indigestion. Equilibrium is established by detection of an excess amount of hydrochloric acid in the stomach and oesophagus, in which the subsequent addition of the active ingredient, Mg(OH)2, thereby shifts the equation to the right, instigating more magnesium hydroxide to dissolve and counteract the acid in neutralisation (Flinn Scientific , 2016). However, this can only be expected to occur if the controlled variables are kept constant, observing tests fair and deducting conclusive results. As variables that are not being measured, examples of these include an unchanged method, the phenolphthalein indicator and use of an unchanged brand and type of antacid.
Table 1: Classification of Variables
| Independent variable | Dependent Variable | Constant variable |
| Amount of antacid with active ingredient of Magnesium Hydroxide – Mg(OH)2 | The amount of sodium hydroxide needed to neutralise in the reaction, calculated through a back titration |
|
2.0 Materials
- 1x Pack of Magnesium 2Go Double Strength Chewable Tablets
- 200mL 0.1M Sodium Hydroxide
- 1x 250mL Conical Flask
- 1x Burette stand and clamp
- 1x Mortar and pestle
- 500mL 0.1M Hydrochloric Acid
- 500mL Deionised Water
- 1x Phenolphthalein Indicator
- 1x pH Probe
- 1x Scales
- 1x Water Bath
- 1x 25mL Glass Pipette
- 1x Plastic 3mL Pipette
- 1x 50mL measuring cylinder
- 1x Glass Thermometer
- 1x Weighing boat
3.0 Method
Step 1: Materials required for the experiment (found in section 2.0) were retrieved and set up on the lab bench in preparation.
Step 2: The pH probe was connected via Bluetooth to the laptop being used for data collection.
Step 3: The pH probe was calibrated by testing the pH’s of differing calibration liquids in the calibration section of the PASCO Capstone programme.
Step 4: The water bath was set up at 37oC.
Step 4: One antacid tablet was crushed with a mortar and pestle.
Step 5: The crushed tablet was weighed with an electronic scale on a weighing boat.
Step 6: The crushed tablet was divided into two portions of equal amounts to represent the effects of half an antacid tablet.
Step 7: Using distilled water, the antacid was made up into 20mLs of water and the pH was tested.
Step 8: 20mL of 0.1M Hydrochloric Acid (HCl) was measured into a conical flask.
Step 9: The 20mLs of both HCl and antacid-water were combined in the conical flask, making a 40mL solution.
Step 10: The analyte, was transferred into sample cups and was put in the water bath until heated to 37oC.
Step 11: Using a glass pipette, 20mL of Sodium Hydroxide was measured and transferred into the burette.
Step 12: The analyte was removed from the water bath and the pH tested.
Step 13: 3 drops of the phenolphthalein indicator was added to the solution.
Step 14: The conical flask was placed under the burette and the back titration began.
Step 15: Sodium Hydroxide (Na(OH)2) was gradually added into the solution until the end point was reached, indicated by the colour change of the solution from colourless to pink.
Step 16: Data was taken, in regards to the volume left in the burette once the end point had been reached.
Step 17: The final solution, including the antacid, HCl and Na(OH)2 was tested for its pH to conclude that the solution was indeed, neutral.
Step 18: Repeat steps 4-18 with varying amounts of antacid, for a whole tablet (2.2g), a tablet and a half (3.3g), and two tablets (4.5g).
4.0 Preliminary Investigations
Preliminary investigations were undertaken in order to achieve an experimental design and process with fewer unsubstantiated assumptions, as well as to attain a general understanding of whether the experiment will produce results of which coincide with the values hypothesised. The results of these preliminary trials can be found below in tables 2-4.
Table 2: Results of Preliminary Test 1
| Amount of Tablet | Mass (g) | Volume of NaOH | pH prior to HCl | pH with HCl |
| ½ Tablet | 1.1g | 4.2mL | 8.81 | 2.11 |
| 1 Tablet | 2.28g | 0.4mL | 8.86 | 2.16 |
| 1 ½ Tablets | 3.32g | 0.2mL | 8.96 | 4.17 |
| 2 Tablets | 4.58g | 0.1mL | 9.01 | 4.52 |
Table 3: Results of Preliminary Test 2
| Amount of Tablet | Mass (g) | Volume of NaOH |
| ½ Tablet | 1.1g | 1mL |
| 1 Tablet | 2.25g | 1.1mL |
| 1 ½ Tablets | 3.37g | 1mL |
| 2 Tablets | 4.52g | 0.2mL |
| Amount of Tablet | Mass (g) | Volume of NaOH | pH prior to HCl | pH with HCl |
| ½ Tablet | 1.1g | 5.4mL | 8.73 | 2.03 |
| 1 Tablet | 2.3g | 2.6mL | 8.89 | 2.19 |
| 1 ½ Tablets | 3.36g | 1.2mL | 8.98 | 4.19 |
| 2 Tablets | 4.57g | 0.6mL | 9.12 | 4.63 |
Table 4: Results of Preliminary Test 3
The preliminary trials consisted of three tests, each with slightly differing experimental processes and design in order to achieve a final method apt for the experimental trials. The initial preliminary experiment trialled built the foundation for the remaining preliminary trials, as well as the final experiment process and design of the investigation. However, some specific details has to be manipulated for the investigation to proceed with constant variables.
Within the first preliminary test (results found in table 2), usable and quantitative data of which supported the hypothesis was attained. It was expected that as the amount of antacid added into the solution was increased, that there would be a smaller volume of sodium hydroxide needed to reach the endpoint of the titration. This is shown in the decrease from the 4.2mL of NaOH needed to neutralise half a tablet (1.1g) in 20mL of HCl, to the 0.1mL needed to neutralise two tablets (4.58g) in 20mL of HCl. It was also expected that in testing the pH of the antacid solution (20mL of antacid and distilled water), that the pH would be of alkaline proportions. This is quantified in the measurements of pH’s ranging from 8.81 to 9.01. Likewise, after adding the hydrochloric acid, it was expected that the pH would decrease dramatically, and thus be of a pH between 2 and 4, as this approximately the pH of stomach acid, only marginally higher because of the antacid which has begun to neutralise the acid. In these results, it was also expected that the pH would be higher when higher masses of antacid are in the acid. The pH drops by approximately 6 values for half a tablet and a whole tablet, as compared to between 4.5 and 5 values for one and a half tablets and two tablets. Therefore, the hypothesis was supported. However, a great error was made in the experimental method of the first preliminary trial. In preparing for the titrations, the analyte made up of the specific masses of the antacid, 20mL of distilled water and 20mL of hydrochloric acid was combined two days prior to the titrations. This obscures the data because the solution had approximately 48 hours to react, and showed large difference in results to that of preliminary trial two.
Due to the successive results attained from preliminary trial one, preliminary trial two proceeded with mainly the same method. One of the only differing aspects between the two trials was the time over which they were carried out. Rather than leaving the analyte to react for two days due to time restrictions, the analyte was combined within the same hour the titrations were carried out. This resulted in virtually no difference between samples, meaning the substances within the analyte had not reacted. The samples of half a tablet, a whole tablet, and a tablet and a half remained between 1 and 1.1mL needed to titrate the solution. This can be put down to magnesium hydroxide’s limited solubility, and research of which shows that antacids take approximately 30 to 60 minutes to begin to neutralise. Rather than dissolving wholly and all at once, the compound slowly dissolves as it neutralises the acid. However, due to limited equipment, the pH was not able to be tested to support this theory. Thus, because the results did not support the hypothesis, the modification of heating the analyte was made.
Within preliminary test 3, the modification was made to test whether heating the analyte would present conclusive results in a manner which supports the hypothesis. The analyte solution, made in the same hour as the occurrence of the titration was placed in a water bath at 37oC to replicate the human body’s interior homeostatic temperature. Upon reaching 37oC, the solution was removed from the water bath and titrated immediately as to not lose too much heat to the environment. In titrating the analyte after it was heated, conclusive evidence was attained in the manner that the heat obviously sped up the reaction, based on kinetic energy laws, which state that heat provides energy to particles resulting in a larger number or high energy collisions and speeding up a reaction (Florida State University of Chemistry and Biochemistry, 2018). The results of the titration thus were conclusive in supporting the hypothesis by decreasing in the amount of sodium hydroxide needed to reach the endpoint of the titration, from 5.4mL for half a tablet, to 0.6mL for two tablets.
The modification of heating the analyte solution was added to the final experimental method following this revelation in the preliminary study. Additionally, the only other modification made to the original plan was when the pH should be tested within the experiment. While in testing the pH prior and post adding the HCl showed that the hydrochloric acid changed the pH of the analyte, it did not provide any major evidence of which would support the hypothesis. Thus, the modification was made to test the pH after heating the analyte solution, and again after titration. However, the method of testing the pH before hydrochloric acid was added was kept as it provides data of which can be used in calculating the concentration of OH–ions within the magnesium hydroxide.This will evidentially support the hypothesis as it shows that the addition of sodium hydroxide aids in the neutralisation of the hydrochloric acid. These experimental modifications provide a suitable experimental method suitable for collection of data.
5.0 Risk Assessments
Prior to beginning any experiment, a thorough plan of all procedures involving potential hazards must be completed in order to assure that these hazards have been identified and the risks of such have been assessed to ensure safety (see appendix 1). In this experiment, there are hazardous risks, which conform to specific safety requirements in the application and use of chemicals, glassware and electronic equipment.
The execution of titration techniques is complete with the equipment of a burette, clamp and a conical flask, which in addition can bring dangerous situations. The burette and conical flask, both made of glass, present the possibility of breakage or cracking and thus a danger of cuts or abrasions. Likewise can be said for the use of the mortar and pestle. Subsequently, the inspection of burettes, conical flasks and the mortar and pestle used should be carried out to ensure there is no damage prior to the use of equipment, and discard any damaged equipment. To ensure there is no breakage of equipment throughout the experiment, careful precision of movement and placement of equipment should be monitored. In addition, the burette should be used for scientific purposes only, and specific care should be taken when setting up the experiment especially in regards to the burette clamp, as to not cause breakage of glass, once again, subsequent in spillage of liquids. Spillage of liquids, caused by knocked over conical flasks, measuring cylinders or burettes can create the hazard of slipping on the liquid if not cleaned up immediately and thoroughly.
Similarly, the use of the water bath to heat the antacid, distilled water and hydrochloric acid solution certainly creates potential hazards. The water should only be heated to the set degree, and careful precautions surrounding the water bath should be taken as to not cause spillage of water or burns to the skin. Likewise, the water bath should only be filled to the extent of enough water to surround the object being heated; again, as to not cause a spillage of water. Spilling liquids, while creating a slipping hazard, can also cause potential electrical hazards. Thus, it should be ensured that precaution is taken around the water bath as to not spill any liquid, and should be immediately cleaned if occurs. This precaution should be taken in the case of the electronic balance, also. Additionally for both electronic pieces of equipment, it should be ensured that a check of electrical safety precedes any experimental use. Finally, in the use of a pH electrode, there is the possible hazard of broken glass electrodes and cross-contamination. The probe should be inspected and thoroughly cleaned before use as to not contaminate any solutions throughout the experiment, and also to check for damage to the glass electrode.
In the case of chemicals to be used and produced, hydrochloric acid, magnesium hydroxide, sodium hydroxide and phenolphthalein are all relevant. Hydrochloric acid (HCl), while not classified as a hazardous chemical, still pertains in dangerous circumstances, including irritation of eyes, lungs and skin. Similarly, magnesium hydroxide (Mg(OH)2) may cause skin irritation, serious eye irritation, and may cause respiratory irritation if inhaled. Additionally, sodium hydroxide (NaOH), also not categorised as a hazardous chemical, may cause irritation of the eyes. Lastly, phenolphthalein presents hazards of flammability, serious eye irritation, and skin irritation in the event of prolonged contact. In the use of all chemicals listed above, safety precautions should be taken. Avoiding inhalation of all liquids, vapours and gases, as well as avoiding (as much as possible) contact with skin and eyes are precautions of which should be taken in all aspects of the experiment. Contact with eyes can be avoided by wearing safety glasses, and if any chemicals come into contact with the eyes, they should be flushed with water immediately. None of the chemicals, including water, within the laboratory should be consumed, and all liquid spills should be cleaned immediately. After use of equipment and chemicals, benches of which have been used should be wiped down, and hands should be washed thoroughly with soap and water. In taking the precautions listed for the potential hazards above, the experiment should run smoothly, and safety and health of all participants should remain as it was.
6.0 Results
Table 5: Experiment 1
| Test | Grams of Antacid | Volume of NaOH (mL) | pH Before HCl was Added | pH Prior to Back Titration | pH Post Back Titration |
| 1 (½ tab.) | 1.125g | 4mL | 8.86 | 3.39 | 6.78 |
| 2 (1 tab.) | 2.27g | 1.9mL | 8.9 | 3.95 | 6.9 |
| 3 (1½ tab.) | 3.38g | 0.6mL | 8.94 | 4.16 | 7.54 |
| 4 (2 tab.) | 4.57g | 0.7mL | 9.0 | 4.66 | 7.83 |
Table 6: Experiment 2
| Test | Grams of Antacid | Volume of NaOH (mL) | pH Before HCl was Added | pH Prior to Back Titration | pH Post Back Titration |
| 1 (½ tab.) | 1.125g | 7.9mL | 8.80 | 2.9 | 6.21 |
| 2 (1 tab.) | 2.25g | 3.3mL | 8.86 | 4.1 | 6.89 |
| 3 (1½ tab.) | 3.39g | 0.9mL | 8.96 | 4.2 | 7.0 |
| 4 (2 tab.) | 4.50g | 1.5mL | 9.06 | 4.50 | 7.6 |
Table 7: Experiment 3
| Test | Grams of Antacid | Volume of NaOH (mL) | pH Before HCl was Added | pH Prior to Back Titration | pH Post Back Titration |
| 1 (½ tab.) | 1.14g | 4.7mL | 8.78 | 4.1 | 7.1 |
| 2 (1 tab.) | 2.29g | 2.4mL | 8.82 | 4.4 | 7.2 |
| 3 (1½ tab.) | 3.44g | 0.8mL | 8.98 | 5.5 | 7.5 |
| 4 (2 tab.) | 4.63g | 0.6mL | 9.12 | 6.3 | 7.9 |
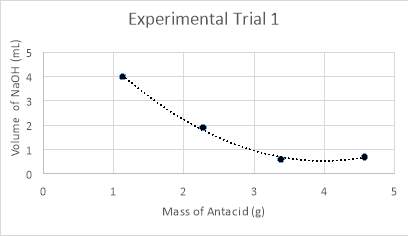
Graph 1: Experiment 1 – Mass vs. Volume
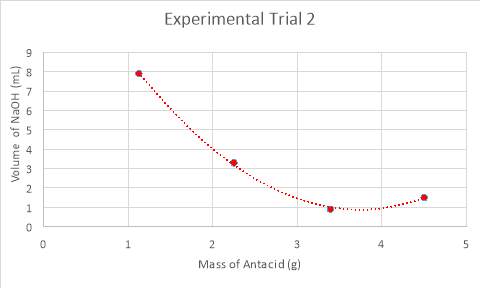
Graph 2: Experiment 2 – Mass vs, Volume
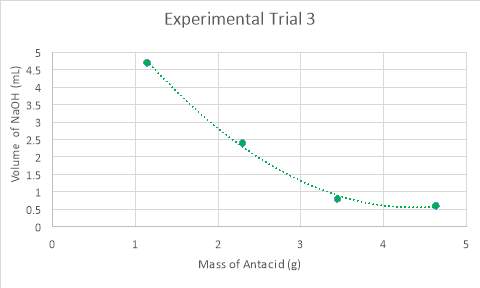
Graph 3: Experiment 3 – Mass vs. Volume
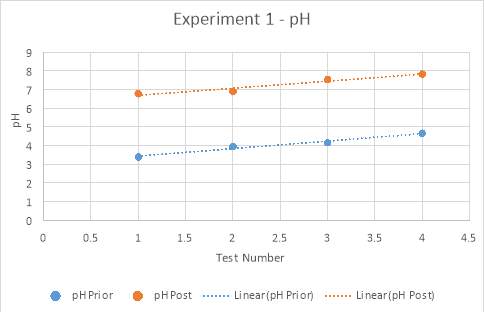
Graph 4: Experiment 1 – pH prior and post titration
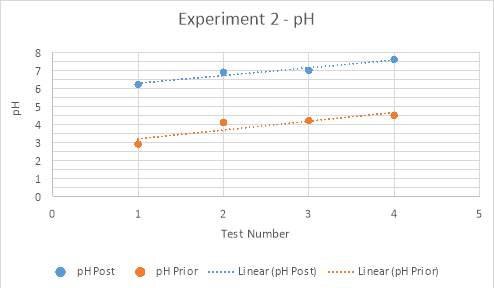
Graph 5: Experiment 2 – pH prior and post titration
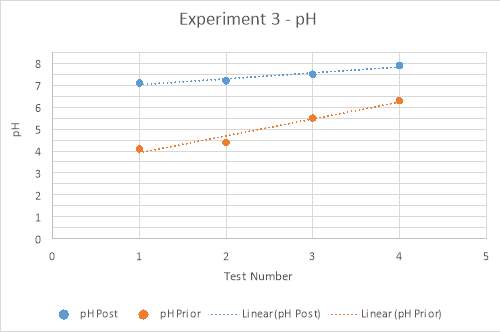
Graph 6: Experiment 3 – pH prior and post titration
7.0 Processing of the Data
The below values are secondary data, produced through evidentiary manipulation of raw data. The calculations of the values can be found in section 12.1 (appendix).
Table 8: Average mass
| Amount of Tablet | Average Mass (g) |
| ½ Tablet | 1.13 |
| 1 Tablet | 2.27 |
| 1 ½ Tablets | 3.403 |
| 2 Tablets | 4.567 |
Table 9: Average volume of NaOH
| Amount of Tablet | Average volume of NaOH (mL) |
| ½ Tablet | 5.53 |
| 1 Tablet | 1.267 |
| 1 ½ Tablets | 0.767 |
| 2 Tablets | 0.93 |
Table 10: Uncertainty for mean of mass
| Amount of Tablet | Uncertainty |
| ½ Tablet | 0.0075 |
| 1 Tablet | 0.02 |
| 1 ½ Tablets | 0.03 |
| 2 Tablets | 0.065 |
Table 11: Uncertainty for mean of volume
| Amount of Tablet | Uncertainty |
| ½ Tablet | 1.95 |
| 1 Tablet | 0.7 |
| 1 ½ Tablets | 0.15 |
| 2 Tablets | 0.45 |
Table 12: Percentage Uncertainty for mass
| Amount of Tablet | Percentage Uncertainty |
| ½ Tablet | 0.22% |
| 1 Tablet | 0.29% |
| 1 ½ Tablets | 0.29% |
| 2 Tablets | 0.47% |
Table 13: Percentage Uncertainty for volume
| Amount of Tablet | Percentage Uncertainty |
| ½ Tablet | 11.75% |
| 1 Tablet | 9.21% |
| 1 ½ Tablets | 6.52% |
| 2 Tablets | 16.07% |
8.0 Analysis of Results and Discussion
The experiment conducted was evaluated to investigate how much antacid is needed to neutralise stomach acid in regards to the biochemical process of indigestion. It was hypothesised that as the amount of magnesium hydroxide antacid (measured in grams) increases from a dose of half a tablet (1.13g) to two tablets (4.567g), less sodium hydroxide will be required to neutralise the hydrochloric acid. This experiment was investigated through specific attention and detail in regards to the chemical processes of acid-base reactions, through concepts of the Bronsted-Lowry theory, neutralisation, and equilibrium in a chemical reaction. A comprehensive understanding of these chemical concepts allow for a thorough analysis of the experimental process, in which provide theoretical and experimental evidence as to why the results partially supported the hypothesis.
The raw, primary data collected and displayed in Tables 5-7 explicitly demonstrates the parallels between the mass of antacid in the analyte and the volume of sodium hydroxide needed to neutralise the solution, in addition to the corresponding pH prior and post titration. Likewise, Graphs 1-3 aggregately and visually represent the data, and present trends within the data of which display a polynomial development. A comparison of all three experiments can be seen in graph 7, below. Additionally, Graphs 4-6 visually present the data of the pH of the analyte, tested after heating (step 12 in method) and following the completion of the titration (step 17 in method), which similarly support the hypothesis in quantifiably measuring the change in pH to confirm the solution is neutral at its titrated endpoint. A comparison of these results is seen on graph 8, below. These quantitative results can be further explained through various chemical concepts, and individual analysis of each experimental trial.
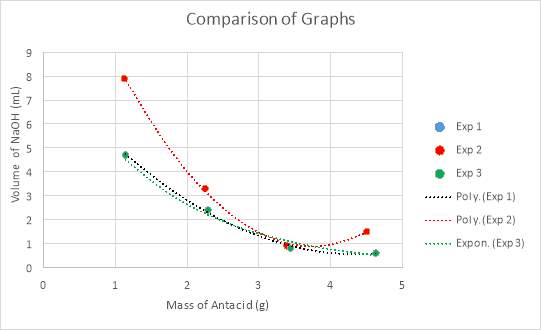
Graph 7: Comparison of all three experimental trials, in regards to the mass of antacid versus the volume of NaOH required for neutralisation.
Graph 7, displayed above, compares all three trials by their mass of antacid in the analyte and the volume of NaOH required to neutralise the analyte. It can be deduced from an analysis of the data in Tables 5-7, in conjunction with visual results in the graph, that the data takes form in a negative slope, in ether a polynomial or exponential form. The reason for differing forms of trendlines is the results of the experiments. Experiments one and two refuted the hypothesis in the manner that the lowest volume needed to neutralise the solution was completed with one and a half tablets, rather than the hypothesised two tablets. Thus, for the analysis of experiments one and two, a polynomial trendline was used as rather than beginning a plateau or continuing a negative slope, begins to curve upwards, as would a polynomial function. This can be considered a good fit for the data, as the R2 value, otherwise known as the coefficient of determination, of which statistically measures how close the data is to the regression line is of 0.99. In looking at this value, it can be determined that it is a good fit for the data as it is extremely close to one. However, an anomaly is present within experimental trial two, in testing half a tablet. Despite not explicitly presenting itself as an anomaly on the graph (graph 2), an analysis of the data and close inspection of the graph reveals otherwise. While the mass is exactly the same to that in the test before, it required more than two millilitres more when neutralising with sodium hydroxide. Additionally, while the mass for experimental trial three is higher, this mass still does not require as high of a volume of NaOH as the second trial. Conversely, experimental trial three supported the hypothesis and corroborated with the quantitative results laid foundationally in the preliminary trials. There is a certain point of neutralisation and equilibrium at which the reaction would begin reversing and becoming too alkaline for the conditions of the stomach, which is past the pH of eight. Thus, it would be expected that as the amount of magnesium hydroxide added increases, that the volume of sodium hydroxide needed to neutralise the analyte would plateau. This can be seen in an exponential function, and within the data, where it can be seen that when experimenting with two tablets, that the data is beginning to plateau.
Graph 8, displayed below, compares the pH’s of each experimental trial prior to and post titration. The data retrieved in all experiments concerning the pH of the solution supports the hypothesis. It was theorised that prior to titration, the analyte would have a low, acidic pH, increasing with the amount of magnesium hydroxide added. Furthermore, proceeding the titration, it was hypothesised that the pH would be reaching neutral proportions, between a pH of six to eight, again, increasing with the amount of magnesium hydroxide added. The data attained in the form of pH values had only few major anomalies, of which may have prospectively altered the average pH for this test number across all three experiments. Viewed visually in Graph 5 and Table 6, the pH recorded for test number two is higher than what is expected of the linear function. Similarly, viewing the comparison graph, it can be seen that the pH prior to titration in experimental trial three is, on average, of much higher value than the other two experiments conducted. This anomaly could be concluded to have been an error in the pH probe, or dissatisfactory cleaning of the probe after previous use. However, this data was attained and thus presents a smaller difference in pH before and after titration. From the fourth test in trial three, it can be calculated that the difference in pH is only 1.6. In comparison to the fourth tests from experimental trials one and two, there is a clear difference in that they both have differences in pH of approximately 3.1. Additionally, the values attained from experimental trial three’s prior pH’s presents an anomaly in the manner that it is conspicuous in its rate of increase, which although is constant, grants a larger gradient. Evidence of this can be seen on graphs 4-6, whereby Experiment one and two’s prior pH’s have gradients of approximately 0.4, as compared to experiment three’s gradient of 0.77.
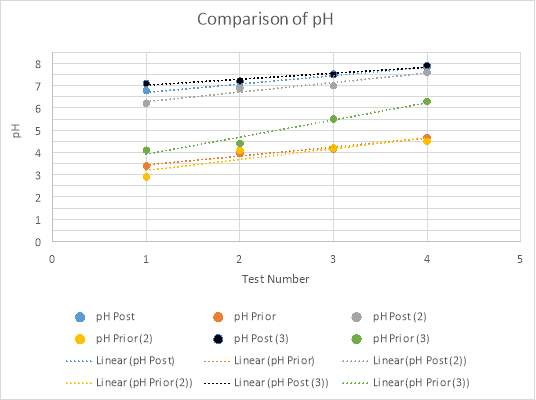 Graph 8: Comparison of all three experimental trials, in regards to the pH prior and post titration.
Graph 8: Comparison of all three experimental trials, in regards to the pH prior and post titration.
There is a perceptible commonality however, in the linear functions of all pH’s tested, both prior and post titration. This is the positive slant the function travels in, supporting the hypothesis through the indication that as more magnesium hydroxide is added into the analyte with each test, the pH increases. This is due to the neutralisation process being undergone by the base, magnesium hydroxide, and the acid, hydrochloric acid.
Within the experiment, one of the first steps within the method is to combine an antacid tablet, of which the active ingredient is magnesium hydroxide, with hydrochloric acid. Magnesium hydroxide, while it is limited in solubility, produces low concentrations of hydroxides. Thus, while chemistry may categorise the base as strong because of the ‘OH’ found in its chemical compound, Mg(OH)2, the base acts as if it is one of weak alkalinity (Saylor Education, 2018). Therefore, within the reaction of HCl and Mg(OH)2 (shown below), the neutralisation resulted in a pH’s less than seven.
Mg(OH)2+2HCl →MgCl2+2H2O
It can deduced that this is not fully neutralised, and thus sodium hydroxide was used in a back titration process. These processes are used in experiments of which the solid is insoluble or only partially soluble in water. Magnesium hydroxide’s solubility constant (Ksp) is approximately 1.5×10-11, meaning that while the compound can react with the hydrochloric acid, it did so slowly (Massachusetts Institute of Technology, 2010). Due to the neutralisation reaction between the components of magnesium hydroxide (Mg(OH)2) and hydrochloric acid (HCl), water and magnesium chloride are formed subsequently as a products. However, in this formation of water, some of the OH–ions are used, thus shifting the reaction to the right as the ions are used up. Experimentally, it was found that ½ a tablet and a whole tablet have a hydroxide concentration of 3×10—9, as compared to 1 ½ tablets and two tablets, which had concentrations of 2×10-9 (see appendix 12.1). This causes more of the concentration of magnesium hydroxide to dissolve into the hydrochloric acid, and split into its dissociative ions. This is due to Le Chatelier’s principle, whereby as more hydroxide ions are removed because of their bonding with H+ions in the acid to form hydronium ions (H3O+), magnesium hydroxide is forced to dissociate further to replace said ions (Chemistry LibreTexts, 2018). Here, it can be said that the process is following the Bronsted-Lowry theory of acids and bases, where the strong hydrochloric acid is acting as a proton donor, whereby the OH–ions from the weak magnesium hydroxide are acting as a proton acceptor.
This process of neutralisation in accordance with equilibrium processes of the reaction, theorised by Le Chatelier, supports the hypothesis. As the base in the form of Mg(OH)2is increased in concentration through the amount of antacid tablet added, the reaction is shifted to the left, until a new equilibrium is restored by the formation of water and salt from the two reactants, in which case more antacid is dissolved (Haustein, 2014). However, this is only partially supported by data, because of the previously mentioned increase in volume for test four in both experiment one and two. The pH presents a distinguishable commonality between each test, excluding the anomaly discussed, which likewise supports the hypothesis.
While the experimental investigation produced successful conclusions and results, leading to the partial support of the hypothesis, errors and limitations within the experimental process were unavoidable, as they are in all scientific investigations. Calculations of percentage uncertainty (see appendix 12.1) display a range of values which display the ambiguity of the results, and thus a quantitative calculation of the factors of error. Through measuring the mass of the amount of each tablet, it can be established that there is the opportunity and probability of error, which is quantified in said calculations. While the percentages are all less than 1%, there is still a possibility that throughout the experiment some of the mass was lost in extenuating circumstances. For example, as the solubility of magnesium hydroxide is low, it is possible that in transferring the analyte containing the compound from one piece of equipment to another (i.e. sample cup to conical flask), some of the mass remained in the first piece of equipment. Likewise, as the substance was crushed into a loose powder, transferral from the weighing boat into the sample cup may have lost some of the particles to the environment. Similarly, errors may have occurred in the process of titration, again, quantified in the percentage uncertainty calculations. These errors may have transpired in the reading of initial and final values of the titration. Additionally, errors and inaccuracies of the electronic pH probes used must be accounted for; which may have occurred due to lack of satisfactory cleaning from other experiments, and between each test, as well as functional errors within the probes. However, whilst errors such as those listed above may have occurred, the percentage uncertainty calculations in conjunction with the analysis and discussion of errors provides logical and quantitative evidence and reasoning for errors, which while should be considered, have been accounted for in the process.
The unification of both theoretical processes of chemistry and the experimental findings analysed presents partial support of the hypothesis, which stated:
It was hypothesised that as the amount (measured in grams) of antacid with the active ingredient of magnesium hydroxide (Mg(OH)2) increases from approximately 1.1g to 4.5g (12 a tablet to 2 tablets), the hydrochloric acid (HCl) will be neutralised with less sodium hydroxide in a back titration. Thus, pushing the equilibrium reaction to the right, moving the pH of the solution closer to seven.
9.0 Recommendations and Conclusion
Throughout the experimental process, there were aspects of the method and equipment used that could be improved upon if the experiment were to be completed again in future investigations. In order to attain a more precise representation of the data and prospectively, full support of the hypothesis, it is suggested that more experimental trials should be taken, with more tests of amounts of the antacid in each experiment. This modification would contribute to the assurance that precise data is collected, and of such can provide a more exact representation of the results, giving a wider range for averages and calculations of error to be taken from. Furthermore, it is suggested that a comparison of differing antacids within the experiment is investigated, of which would provide a further comprehension of the ability of differentiating active ingredients in the form of weak bases to neutralise a strong acid such as that of hydrochloric acid.
Indigestion as an internal physical process of human biology effects people worldwide, and while it may be an easily fixed problem, it may have either short or long-term effects. Expunging the heartburn through consumption of antacid tablets with the active ingredient of magnesium hydroxide has been proven through this experimental investigation to be a solution to neutralising the stomach acid in the form of hydrochloric acid. Overall, it can be concluded that the data attained from this investigation mainly supports the hypothesis, explained through theories and concepts of equilibria and acid-base chemistry, having fundamental impacts on processes within the human body, such as indigestion.
10.0 Acknowledgments
I would like to acknowledge Mr. Gooley for providing vital foundational chemical concepts of which apply to theories of acid-base experiments and equilibria. I would also like to thank Ms. Philippa for aiding me in the experimental process of the investigation.
11.0 Bibliography
Chemistry LibreTexts. (2018, May). Neutralization. Retrieved from Chemistry LibreTexts: https://chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Acids_and_Bases/Acid%2F%2FBase_Reactions/Neutralization
Clark, J. (2013, November). Strong and Weak Bases. Retrieved from Chemguide: https://www.chemguide.co.uk/physical/acidbaseeqia/bases.html
Crampton, L. (2017, October). Hydrochloric Acid in the Stomach and Digestive Problems. Retrieved from Owlcation: https://owlcation.com/stem/Hydrochloric-Acid-in-the-Stomach-and-Digestive-Problems
Eddy, D. (2016). Chemistry 104: Analysis of Commercial Antacid Tablets. Retrieved from Louisiana Tech University: http://www.chem.latech.edu/~deddy/chem104/104Antacid.htm
Ellesmere OCR. (2015). Bronsted-Lowrry Acids and Bases. Retrieved from Ellesmere OCR A level Chemistry: https://sites.google.com/site/ellesmerealevelchemistry/module-5-physical-chemistry-transition-elements/5-1-rates-equilibrium-and-ph/5-1-3-acids-bases-and-buffers/5-1-3-a-broensted-lowry-acids-and-bases
Flinn Scientific . (2016). Neutralisation Reaction of an Antacid. Retrieved from Flinn Scientific: https://www.flinnsci.com/api/library/Download/a4c91d864e3e47f3b213a523ef2d55c4
Flinn Scientific. (2016). Neutralization Reaction of an Antacid. Retrieved from Flinn Scientific: https://www.flinnsci.com/api/library/Download/a4c91d864e3e47f3b213a523ef2d55c4
Florida State University of Chemistry and Biochemistry. (2018). Reaction Rates. Retrieved from Florida State University of Chemistry and Biochemistry: https://www.chem.fsu.edu/chemlab/chm1046course/rates.html
FuseSchool. (2013). What is Dynamic Equilibrium? | The Chemistry Journey. Retrieved from FuseSchool – Global Education: https://www.youtube.com/watch?v=wlD_ImYQAgQ
Haustein, C. H. (2014). Neutralization. In L. Lerner, & B. Wilmoth-Lerner, The Gale Encyclopedia of Science: Volume 6, 5th Edition (pp. 3006-3008). Michigan: Cengage Learning.
International Foundation for Functional Gastrointestinal Disorders. (2015, September 4). Antacids. Retrieved from International Foundation for Functional Gastrointestinal Disorders: https://www.iffgd.org/diet-treatments/antacids.html
John Wiley & Sons. (2004). Acids, Bases and pH. Retrieved from Wiley : https://www.wiley.com/college/pratt/0471393878/student/review/acid_base/4_strong_and_weak.html
Johnson & Johnson Pacific Pty Limited. (2014). Mylanta Original. Retrieved from Mylanta: http://www.mylanta.com.au/mylanta-original.html
Khan Academy. (2018). Acids and Bases Topic. Retrieved from Khan Academy: https://www.khanacademy.org/science/chemistry/acids-and-bases-topic/acids-and-bases/a/bronsted-lowry-acid-base-theory
Khan Academy. (2018). Arrhenius Acids and Bases. Retrieved from Khan Academy: https://www.khanacademy.org/science/chemistry/acids-and-bases-topic/acids-and-bases/a/arrhenius-acids-and-bases
Lower, S. (2017, October 28). Le Chatelier Principle: Shifting the Equilibrium. Retrieved from Simon Fraser University: http://www.chem1.com/acad/webtext/chemeq/Eq-02.html
Lumen Learning. (2018). Acids and Bases. Retrieved from Lumen Learning: https://courses.lumenlearning.com/boundless-chemistry/chapter/acids-and-bases/
Lumen Learning. (2018). Factors that Affect Chemical Equilibrium. Retrieved from Lumen Learning: https://courses.lumenlearning.com/boundless-chemistry/chapter/factors-that-affect-chemical-equilibrium/
Mahmood, T. (2018). Concepts of Dynamic and Chemical Equilibrium. Retrieved from IGCSE and IAL Chemistry: https://igcseandialchemistry.com/concepts-dynamic-chemical-equilibrium/
Massachusetts Institute of Technology. (2010, May 6). Magnesium Hydroxide. Retrieved from Massachusetts Institute of Technology: http://web.mit.edu/piuska/Public/Ekaterinburg%20more/Ekaterinburg%20research/Magnesium%20hydroxide%20-%20Wikipedia.pdf
Ogbru, A. (2018). Antacids. Retrieved from RXList: https://www.rxlist.com/antacids/drugs-condition.htm
Ophardt, C. E. (2003). pH Scale. Retrieved from Elmhurst College Vitual Chembook: http://chemistry.elmhurst.edu/vchembook/184ph.html
Purdue University. (2018). Le Chatelier’s Principle. Retrieved from Purdue University: http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch16/lechat.html
Rashe, K., & Peterson, L. (2016, January 18). Solubility Product Constant, Ksp. Retrieved from Chemistry Libre Texts: https://chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Equilibria/Solubilty/Solubility_Product_Constant%2C_Ksp
Rice University. (2015). Chemical Equilibria. Retrieved from Rice University: https://opentextbc.ca/chemistry/chapter/13-1-chemical-equilibria/
Ryan, L. (2018). Strong and Weak Acids. Retrieved from Bangkok Patana School: https://www.patana.ac.th/parents/curriculum/chemistry/units/LR1604.html
Saylor Education. (2018). Strong and Weak Acids and Bases and their Salts. Retrieved from Saylor Education: https://saylordotorg.github.io/text_introductory-chemistry/s16-04-strong-and-weak-acids-and-base.html
Smithsakol, T. (2018). Acids and Bases. Retrieved from Advanced Chemistry: https://advchem-niva.wikispaces.com/Acids+and+Bases
Unknown. (2018). pH Scale Universal Indicator pH Colour Chart . Retrieved from Vector Stock: https://www.vectorstock.com/royalty-free-vector/ph-scale-universal-indicator-ph-color-chart-vector-15761416
Wyman, E. (2018). Review: Acid and Base Strength. Retrieved from Study.com: https://study.com/academy/lesson/acid-base-equilibrium-calculating-the-ka-or-kb-of-a-solution.html#transcriptHeader
12.0 Appendix
12.1 Appendix 1 – Calculations
Average grams:
½ tablet:
1.125+1.125+1.143=1.13
1 tablet:
2.27+2.25+2.293=2.27
1 ½ Tablet:
3.38+3.39+3.443=3.403
2 Tablets:
4.57+4.50+4.633=4.567
Average Volume of NaOH:
½ tablet:
4+7.9+4.73=5.53
1 tablet:
1.9+3.3+2.43=1.267
1 ½ Tablet:
0.6+0.9+0.83=0.767
2 Tablets:
0.7+1.5+0.63=0.93
Uncertainty for Mean (g):
½ tablet:
1.14-1.1252=0.0075
1 tablet:
2.29-2.252=0.02
1 ½ Tablet:
3.44-3.382=0.03
2 Tablets:
4.63-4.502=0.065
Uncertainty for Mean (vol):
½ tablet:
7.9-42=1.95
1 tablet:
3.3-1.92=0.7
1 ½ Tablet:
0.9-0.62=0.15
2 Tablets:
1.5-0.62=0.45
Percentage Uncertainty (g):
½ tablet:
0.00753.39×100=0.22%
1 tablet:
0.026.81×100=0.29%
1 ½ Tablet:
0.0310.21×100=0.29%
2 Tablets:
0.06513.7×100=0.47%
Percentage Uncertainty (vol):
½ tablet:
1.9516.6×100=11.75%
1 tablet:
0.77.6×100=9.21%
1 ½ Tablet:
0.152.3×100=6.52%
2 Tablets:
0.452.8×100=16.07%
pH Calculations:
½ Tablet:
When the pH is 8.81, the concentration of the solution is equal to
1 ×10-8.81. Thus,
pH= -log10[1 ×10-8.81]
The balanced equation for magnesium hydroxide is as follows:
Mg(OH)2←→Mg2++2OH-
Therefore, there is a ratio of 1:1:2. Thus, the concentration of OH–ions is:
=1 ×10-8.81×2
=3 ×10-9M
1 Tablet:
When the pH is 8.86, the concentration of the solution is equal to
1 ×10-8.86. Thus,
pH= -log10[1 ×10-8.86]
The balanced equation for magnesium hydroxide is as follows:
Mg(OH)2←→Mg2++2OH-
Therefore, there is a ratio of 1:1:2. Thus, the concentration of OH–ions is:
=1 ×10-8.86×2
=3 ×10-9M
1 ½ Tablets:
When the pH is 8.96, the concentration of the solution is equal to
1 ×10-8.96. Thus,
pH= -log10[1 ×10-8.96]
The balanced equation for magnesium hydroxide is as follows:
Mg(OH)2←→Mg2++2OH-
Therefore, there is a ratio of 1:1:2. Thus, the concentration of OH–ions is:
=1 ×10-8.96×2
=2 ×10-9M
2 Tablets:
When the pH is 9.06, the concentration of the solution is equal to
1 ×10-9.06. Thus,
pH= -log10[1 ×10-9.06]
The balanced equation for magnesium hydroxide is as follows:
Mg(OH)2←→Mg2++2OH-
Therefore, there is a ratio of 1:1:2. Thus, the concentration of OH–ions is:
=1 ×10-9.06×2
=2 ×10-9M
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allRelated Content
All TagsContent relating to: "Pharmacology"
Pharmacology involves the study of drugs and how they affect the body. A pharmacologist contributes to drug development by researching and testing how the body reacts to medication, and whether the medication can have a positive impact on the body in terms of fighting illness and disease.
Related Articles
DMCA / Removal Request
If you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please:




